|
Reformatskii Reaction
The Reformatsky reaction (sometimes misspelled Reformatskii reaction) is an organic reaction which condenses aldehydes or ketones with α-halo esters using metallic zinc to form β-hydroxy-esters: The organozinc reagent, also called a 'Reformatsky enolate', is prepared by treating an alpha-halo ester with zinc dust. Reformatsky enolates are less reactive than lithium enolates or Grignard reagents and hence nucleophilic addition to the ester group does not occur. The reaction was discovered by Sergey Nikolaevich Reformatsky. Some reviews have been published. In addition to aldehydes and ketones, it has also been shown that the Reformatsky enolate is able to react with acid chlorides, imines, nitriles (see Blaise reaction), and nitrones. Moreover, metals other than zinc have also been used, including magnesium, iron, cobalt, nickel, germanium, cadmium, indium, barium, and cerium. Additionally, metal salts are also applicable in place of metals, notably samarium(II) iodide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sergey Reformatsky
Sergey Nikolaevich Reformatsky (russian: Серге́й Никола́евич Реформа́тский) (April 1, 1860 – July 28, 1934) was a Russian chemist. Life He was born as a son of a preacher in Borisoglebskoe, near Ivanovo. He studied at the University of Kazan under Alexander Mikhailovich Zaitsev until 1882. He went to Germany for further studies. He joined Victor Meyer at the University of Heidelberg and Wilhelm Ostwald at the University of Leipzig and finally getting his Ph.D in 1891. The following year he was appointed professor at the University of Kyiv where he stayed the rest of his life. Work In 1887 discovered the Reformatsky reaction, during which a zinc organic compound is the key component. The use of zinc in organic reactions was common at that time, but it was subsequently replaced by the more convenient magnesium. This was not possible for the reaction of α-chloro acids with ketones, because the magnesium based Grignard reagent A Grignard rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in front of oxygen (32.1% and 30.1%, respectively), forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust. In its metallic state, iron is rare in the Earth's crust, limited mainly to deposition by meteorites. Iron ores, by contrast, are among the most abundant in the Earth's crust, although extracting usable metal from them requires kilns or furnaces capable of reaching or higher, about higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BCE and the use of iron tools and weapons began to displace copper alloys, in some regions, only around 1200 BCE. That event is considered the transition from the Bronze Age to the Iron A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry Letters
''Chemistry Letters'' is a peer-reviewed scientific journal published by the Chemical Society of Japan. It specializes in the rapid publication of reviews and letters on all areas of chemistry. The editor-in-chief is Mitsuhiko Shionoya (University of Tokyo). According to the ''Journal Citation Reports'', the journal has a 2014 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as i ... of 1.23. References External links * Chemistry journals Publications established in 1972 English-language journals Academic journals published by learned and professional societies Monthly journals Chemical Society of Japan {{chem-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium(II) Chloride
Titanium(II) chloride is the chemical compound with the formula TiCl2. The black solid has been studied only moderately, probably because of its high reactivity. Ti(II) is a strong reducing agent: it has a high affinity for oxygen and reacts irreversibly with water to produce H2. The usual preparation is the thermal disproportionation of TiCl3 at 500 °C. The reaction is driven by the loss of volatile TiCl4: ::2 TiCl3 → TiCl2 + TiCl4 The method is similar to that for the conversion of VCl3 into VCl2 and VCl4. TiCl2 crystallizes as the layered CdI2 structure. Thus, the Ti(II) centers are octahedrally coordinated to six chloride ligands. Derivatives Molecular complexes are known such as TiCl2(chel)2, where chel is DMPE (CH3)2PCH2CH2P(CH3)2 and TMEDA ((CH3)2NCH2CH2N(CH3)2). Such species are prepared by reduction of related Ti(III) and Ti(IV) complexes. Unusual electronic effects have been observed in these species: TiCl2 CH3)2PCH2CH2P(CH3)2sub>2 is paramagnetic with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium(II) Chloride
Chromium(II) chloride describes inorganic compounds with the formula Cr Cl2(H2O)n. The anhydrous solid is white when pure, however commercial samples are often grey or green; it is hygroscopic and readily dissolves in water to give bright blue air-sensitive solutions of the tetrahydrate Cr(H2O)4Cl2. Chromium(II) chloride has no commercial uses but is used on a laboratory-scale for the synthesis of other chromium complexes. Synthesis CrCl2 is produced by reducing chromium(III) chloride either with hydrogen at 500 °C: :2CrCl3 + H2 → 2CrCl2 + 2HCl or by electrolysis. On the laboratory scale, LiAlH4, zinc, and related reductants produce chromous chloride from chromium(III) precursors: :4 CrCl3 + LiAlH4 → 4 CrCl2 + LiCl + AlCl3 + 2 H2 :2 CrCl3 + Zn → 2 CrCl2 + ZnCl2 CrCl2 can also be prepared by treating a solution of chromium(II) acetate with hydrogen chloride: :Cr2(OAc)4 + 4 HCl → 2 CrCl2 + 4 AcOH Treatment of chromium powder with concentrated hydrochloric aci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
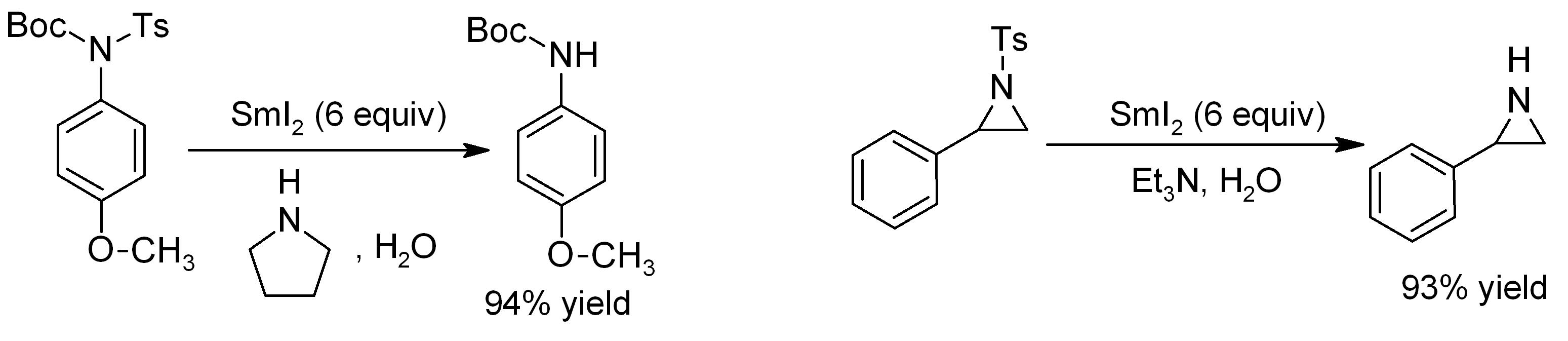
Samarium(II) Iodide
Samarium(II) iodide is an inorganic compound with the formula SmI2. When employed as a solution for organic synthesis, it is known as Kagan's reagent. SmI2 is a green solid and solutions are green as well. It is a strong one-electron reducing agent that is used in organic synthesis. Structure In samarium(II) iodide, the metal centers are seven-coordinate with a face-capped octahedral geometry. In its ether adducts, samarium remains heptacoordinate with five ether and two terminal iodide ligands. Preparation Samarium iodide is easily prepared in nearly quantitative yields from samarium metal and either diiodomethane or 1,2-diiodoethane. When prepared in this way, its solutions is most often used without purification of the inorganic reagent. Solid, solvent-free SmI2 forms by high temperature decomposition of samarium(III) iodide (SmI3).G. Jantsch, N. Skalla: "Zur Kenntnis der Halogenide der seltenen Erden. IV. – Über Samarium(II)jodid und den thermischen Abbau des Samari ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 oxidation state characteristic of the series, it also has a stable +4 state that does not oxidize water. It is also considered one of the rare-earth elements. Cerium has no known biological role in humans but is not particularly toxic, except with intense or continued exposure. Despite always occurring in combination with the other rare-earth elements in minerals such as those of the monazite and bastnäsite groups, cerium is easy to extract from its ores, as it can be distinguished among the lanthanides by its unique ability to be oxidized to the +4 state in aqueous solution. It is the most common of the lanthanides, followed by neodymium, lanthanum, and praseodymium. It is the 25th-most abundant element, making up 66 ppm of the Ear ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element. The most common minerals of barium are baryte ( barium sulfate, BaSO4) and witherite (barium carbonate, BaCO3). The name ''barium'' originates from the alchemical derivative "baryta", from Greek (), meaning 'heavy'. ''Baric'' is the adjectival form of barium. Barium was identified as a new element in 1774, but not reduced to a metal until 1808 with the advent of electrolysis. Barium has few industrial applications. Historically, it was used as a getter for vacuum tubes and in oxide form as the emissive coating on indirectly heated cathodes. It is a component of YBCO (high-temperature superconductors) and electroceramics, and is added to steel and cast iron to reduce the size of carbon grains within the microstructure. Barium compounds ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indium
Indium is a chemical element with the symbol In and atomic number 49. Indium is the softest metal that is not an alkali metal. It is a silvery-white metal that resembles tin in appearance. It is a post-transition metal that makes up 0.21 parts per million of the Earth's crust. Indium has a melting point higher than sodium and gallium, but lower than lithium and tin. Chemically, indium is similar to gallium and thallium, and it is largely intermediate between the two in terms of its properties. Indium was discovered in 1863 by Ferdinand Reich and Hieronymous Theodor Richter by spectroscopic methods. They named it for the indigo blue line in its spectrum. Indium was isolated the next year. Indium is a minor component in zinc sulfide ores and is produced as a byproduct of zinc refinement. It is most notably used in the semiconductor industry, in low-melting-point metal alloys such as solders, in soft-metal high-vacuum seals, and in the production of transparent conductive coati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Like zinc, it demonstrates oxidation state +2 in most of its compounds, and like mercury, it has a lower melting point than the transition metals in groups 3 through 11. Cadmium and its congeners in group 12 are often not considered transition metals, in that they do not have partly filled ''d'' or ''f'' electron shells in the elemental or common oxidation states. The average concentration of cadmium in Earth's crust is between 0.1 and 0.5 parts per million (ppm). It was discovered in 1817 simultaneously by Stromeyer and Hermann, both in Germany, as an impurity in zinc carbonate. Cadmium occurs as a minor component in most zinc ores and is a byproduct of zinc production. Cadmium was used for a long time as a corrosion-resistant plating on steel, and cadmium compounds are used as red, orang ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors silicon and tin. Like silicon, germanium naturally reacts and forms complexes with oxygen in nature. Because it seldom appears in high concentration, germanium was discovered comparatively late in the discovery of the elements. Germanium ranks near fiftieth in relative abundance of the elements in the Earth's crust. In 1869, Dmitri Mendeleev predicted its existence and some of its properties from its position on his periodic table, and called the element ekasilicon. In 1886, Clemens Winkler at Freiberg University found the new element, along with silver and sulfur, in the mineral argyrodite. Winkler named the element after his country, Germany. Germanium is mined primarily from sphalerite (the primary ore of zinc), though germanium is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedron Letters
''Tetrahedron Letters'' is a weekly international journal for rapid publication of full original research papers in the field of organic chemistry. According to the ''Journal Citation Reports'', the journal has a 2020 impact factor of 2.415. Indexing ''Tetrahedron Letters'' is indexed in: References See also *''Tetrahedron In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the o ...'' *'' Tetrahedron: Asymmetry'' Chemistry journals Weekly journals Publications established in 1959 Elsevier academic journals {{chem-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_chloride_tetrahydrate.png)