samarium(II) iodide on:
[Wikipedia]
[Google]
[Amazon]
Samarium(II) iodide is an
 In the Markó-Lam deoxygenation, an alcohol could be almost instantaneously deoxygenated by reducing their toluate ester in presence of SmI2.
:
In the Markó-Lam deoxygenation, an alcohol could be almost instantaneously deoxygenated by reducing their toluate ester in presence of SmI2.
: SmI2 can also be used in the transannulation of bicyclic molecules. An example is the SmI2 induced
SmI2 can also be used in the transannulation of bicyclic molecules. An example is the SmI2 induced  :
The applications of SmI2 have been reviewed. The book ''Organic Synthesis Using Samarium Diiodide'', published in 2009, gives a detailed overview of reactions mediated by SmI2.
:
The applications of SmI2 have been reviewed. The book ''Organic Synthesis Using Samarium Diiodide'', published in 2009, gives a detailed overview of reactions mediated by SmI2.
inorganic compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''.
Inorgan ...
with the formula SmI2. When employed as a solution for organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
, it is known as Kagan's reagent. SmI2 is a green solid and forms a dark blue solution in THF. It is a strong one-electron reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are common reducing agents include hydrogen, carbon ...
that is used in organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
.
Structure
In solid samarium(II) iodide, the metal centers are seven-coordinate with a face-cappedoctahedral geometry
In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The oc ...
.
In its ether adducts, samarium remains heptacoordinate with five ether and two terminal iodide ligands.
Preparation
Samarium iodide is easily prepared in nearly quantitative yields from samarium metal and eitherdiiodomethane
Diiodomethane or methylene iodide, commonly abbreviated "MI", is an organoiodine compound. Diiodomethane is a very dense colorless liquid; however, it decomposes upon exposure to light liberating iodine, which colours samples brownish. It is slig ...
or 1,2-diiodoethane. When prepared in this way, its solutions is most often used without purification of the inorganic reagent.
Solid, solvent-free SmI2 forms by high temperature decomposition
Decomposition is the process by which dead organic substances are broken down into simpler organic or inorganic matter such as carbon dioxide, water, simple sugars and mineral salts. The process is a part of the nutrient cycle and is ess ...
of samarium(III) iodide (SmI3).G. Jantsch, N. Skalla: "Zur Kenntnis der Halogenide der seltenen Erden. IV. – Über Samarium(II)jodid und den thermischen Abbau des Samarium(III)jodids", '' Zeitschrift für Allgemeine und Anorganische Chemie'', 1930, ''193'', 391–405; .'' Gmelins Handbuch der anorganischen Chemie'', System Nr. 39, Band C 6, p. 192–194.
Reactions
Samarium(II) iodide is a powerfulreducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are common reducing agents include hydrogen, carbon ...
– for example it rapidly reduces water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
to hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
. It is available commercially as a dark blue 0.1 M solution in THF. Although used typically in superstoichiometric amounts, catalytic applications have been described.
Organic chemistry
Samarium(II) iodide is a reagent for carbon-carbon bond formation, for example in a Barbier reaction (similar to theGrignard reaction
The Grignard reaction () is an organometallic chemical reaction in which, according to the classical definition, carbon alkyl, allyl, vinyl, or aryl magnesium halides (Grignard reagent) are added to the carbonyl groups of either an aldehyde or ...
) between a ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
and an alkyl iodide to form a tertiary alcohol
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol ...
:
:R1I + R2COR3 → R1R2C(OH)R3
Typical reaction conditions use SmI2 in THF in the presence of catalytic NiI2.
Ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s react similarly (adding two R groups), but aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
s give by-products. The reaction is convenient in that it is often very rapid (5 minutes or less in the cold). Although samarium(II) iodide is considered a powerful single-electron reducing agent, it does display remarkable chemoselectivity Chemoselectivity is the preferential reaction of a chemical reagent with one of two or more different functional groups.
In a chemoselective system, a reagent in the presence of an aldehyde and an ester would mostly target the aldehyde, even if it ...
among functional groups. For example, sulfones and sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. E ...
s can be reduced to the corresponding sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
in the presence of a variety of carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
-containing functionalities (such as ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s, ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s, amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
s, aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
s, etc.). This is presumably due to the considerably slower reaction with carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
s as compared to sulfones and sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. E ...
s. Furthermore, hydrodehalogenation of halogenated hydrocarbons
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic; their odor is usually faint, and may b ...
to the corresponding hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
compound can be achieved using samarium(II) iodide. Also, it can be monitored by the color change that occurs as the dark blue color of SmI2 in THF discharges to a light yellow once the reaction has occurred. The picture shows the dark colour disappearing immediately upon contact with the Barbier reaction mixture.
Work-up is with dilute hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
, and the samarium is removed as aqueous Sm3+.
Carbonyl compounds can also be coupled with simple alkenes to form five, six or eight membered rings.
Tosyl
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or TosIn this article, "Ts", unless otherwise stated, means tosyl, not tennessine.) is a univalent functional group with the chemical formula . It consists of a tolyl ...
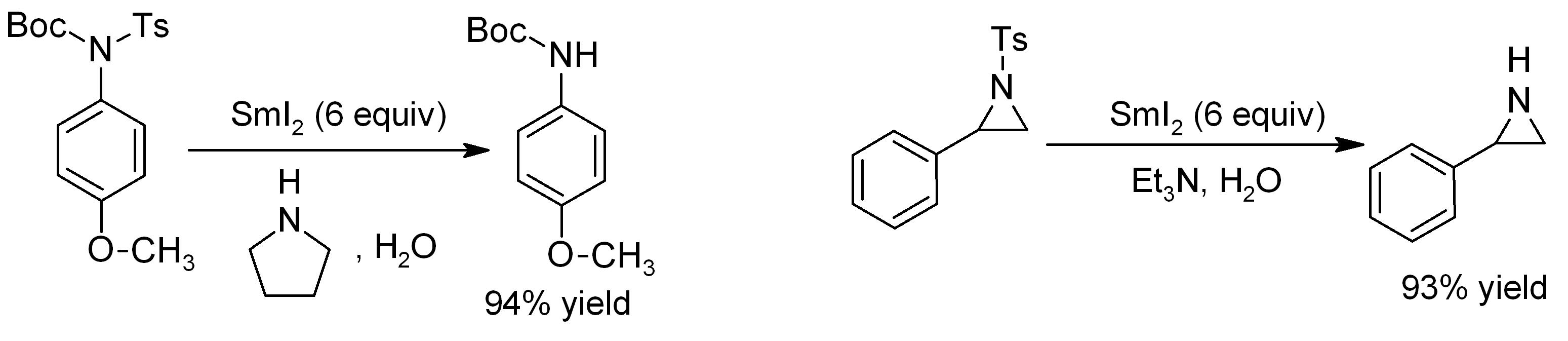
groups can be removed from ''N''-tosylamides almost instantaneously, using SmI2 in conjunction with distilled water and an amine base. The reaction is even effective for deprotection of sensitive substrates such as aziridines:
: In the Markó-Lam deoxygenation, an alcohol could be almost instantaneously deoxygenated by reducing their toluate ester in presence of SmI2.
:
In the Markó-Lam deoxygenation, an alcohol could be almost instantaneously deoxygenated by reducing their toluate ester in presence of SmI2.
:ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
- alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
cyclization
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where ...
of 5-methylenecyclooctanone which proceeds through a ketyl intermediate:
: :
The applications of SmI2 have been reviewed. The book ''Organic Synthesis Using Samarium Diiodide'', published in 2009, gives a detailed overview of reactions mediated by SmI2.
:
The applications of SmI2 have been reviewed. The book ''Organic Synthesis Using Samarium Diiodide'', published in 2009, gives a detailed overview of reactions mediated by SmI2.
References
{{Lanthanide halides Iodides Samarium(II) compounds Lanthanide halides One-electron reducing agents Reagents for organic chemistry