|
Randomized Experiment
In science, randomized experiments are the experiments that allow the greatest reliability and validity of statistical estimates of treatment effects. Randomization-based inference is especially important in experimental design and in survey sampling. Overview In the statistical theory of design of experiments, randomization involves randomly allocating the experimental units across the treatment groups. For example, if an experiment compares a new drug against a standard drug, then the patients should be allocated to either the new drug or to the standard drug control using randomization. Randomized experimentation is ''not'' haphazard. Randomization reduces bias by equalising other factors that have not been explicitly accounted for in the experimental design (according to the law of large numbers). Randomization also produces ignorable designs, which are valuable in model-based statistical inference, especially Bayesian or likelihood-based. In the design of experiments, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flowchart Of Phases Of Parallel Randomized Trial - Modified From CONSORT 2010
A flowchart is a type of diagram that represents a workflow or process. A flowchart can also be defined as a diagrammatic representation of an algorithm, a step-by-step approach to solving a task. The flowchart shows the steps as boxes of various kinds, and their order by connecting the boxes with arrows. This diagrammatic representation illustrates a solution model to a given problem. Flowcharts are used in analyzing, designing, documenting or managing a process or program in various fields. * ''Document flowcharts'', showing controls over a document-flow through a system * ''Data flowcharts'', showing controls over a data-flow in a system * ''System flowcharts'', showing controls at a physical or resource level * ''Program flowchart'', showing the controls in a program within a system Notice that every type of flowchart focuses on some kind of control, rather than on the particular flow itself. However, there are some different classifications. For example, Andrew Veronis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Equipoise
Clinical equipoise, also known as the principle of equipoise, provides the ethical basis for medical research that involves assigning patients to different treatment arms of a clinical trial. The term was first used by Benjamin Freedman in 1987, although references to its use go back to 1795 by Dr. Edward Jenner.Freedman, B. (1987) 'Equipoise and the ethics of clinical research'. ''The New England Journal of Medicine'', 317, (3):141–145. In short, clinical equipoise means that there is genuine uncertainty in the expert medical community over whether a treatment will be beneficial. This applies also for off-label treatments performed before or during their required clinical trials. An ethical dilemma arises in a clinical trial when the investigator(s) begin to believe that the treatment or intervention administered in one arm of the trial is significantly outperforming the other arms. A trial should begin with a null hypothesis, and there should exist no decisive Empirical evidence ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allocation Concealment
In a randomized experiment, allocation concealment hides the sorting of trial participants into treatment groups so that this knowledge cannot be exploited. Adequate allocation concealment serves to prevent study participants from influencing treatment allocations for subjects. Studies with poor allocation concealment (or none at all) are prone to selection bias. Some standard methods of ensuring allocation concealment include sequentially numbered, opaque, sealed envelopes (SNOSE); sequentially numbered containers; pharmacy controlled randomization; and central randomization. CONSORT guidelines recommend that allocation concealment methods be included in a study's protocol, and that the allocation concealment methods be reported in detail in their publication; however, a 2005 study determined that most clinical trials have unclear allocation concealment in their protocols, in their publications, or both. A 2008 study of 146 meta-analyses concluded that the results of randomized cont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
A/B Testing
A/B testing (also known as bucket testing, split-run testing, or split testing) is a user experience research methodology. A/B tests consist of a randomized experiment that usually involves two variants (A and B), although the concept can be also extended to multiple variants of the same variable. It includes application of statistical hypothesis testing or " two-sample hypothesis testing" as used in the field of statistics. A/B testing is a way to compare multiple versions of a single variable, for example by testing a subject's response to variant A against variant B, and determining which of the variants is more effective. Overview "A/B testing" is a shorthand for a simple randomized controlled experiment, in which a number of samples (e.g. A and B) of a single vector-variable are compared. These values are similar except for one variation which might affect a user's behavior. A/B tests are widely considered the simplest form of controlled experiment, especially when they ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Statistical Hypothesis Testing
A statistical hypothesis test is a method of statistical inference used to decide whether the data at hand sufficiently support a particular hypothesis. Hypothesis testing allows us to make probabilistic statements about population parameters. History Early use While hypothesis testing was popularized early in the 20th century, early forms were used in the 1700s. The first use is credited to John Arbuthnot (1710), followed by Pierre-Simon Laplace (1770s), in analyzing the human sex ratio at birth; see . Modern origins and early controversy Modern significance testing is largely the product of Karl Pearson ( ''p''-value, Pearson's chi-squared test), William Sealy Gosset ( Student's t-distribution), and Ronald Fisher ("null hypothesis", analysis of variance, "significance test"), while hypothesis testing was developed by Jerzy Neyman and Egon Pearson (son of Karl). Ronald Fisher began his life in statistics as a Bayesian (Zabell 1992), but Fisher soon grew disenchanted with t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
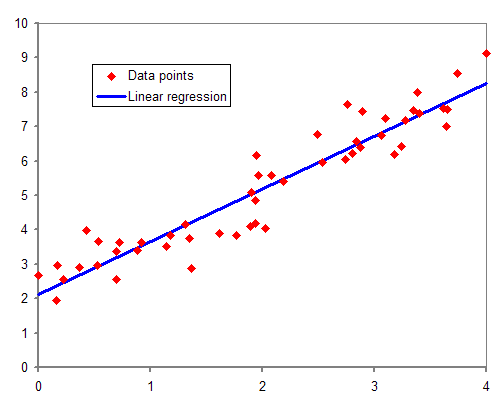
Regression Analysis
In statistical modeling, regression analysis is a set of statistical processes for estimating the relationships between a dependent variable (often called the 'outcome' or 'response' variable, or a 'label' in machine learning parlance) and one or more independent variables (often called 'predictors', 'covariates', 'explanatory variables' or 'features'). The most common form of regression analysis is linear regression, in which one finds the line (or a more complex linear combination) that most closely fits the data according to a specific mathematical criterion. For example, the method of ordinary least squares computes the unique line (or hyperplane) that minimizes the sum of squared differences between the true data and that line (or hyperplane). For specific mathematical reasons (see linear regression), this allows the researcher to estimate the conditional expectation (or population average value) of the dependent variable when the independent variables take on a given ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
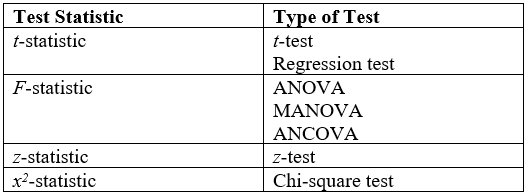
Student's T-test
A ''t''-test is any statistical hypothesis test in which the test statistic follows a Student's ''t''-distribution under the null hypothesis. It is most commonly applied when the test statistic would follow a normal distribution if the value of a Scale parameter, scaling term in the test statistic were known (typically, the scaling term is unknown and therefore a nuisance parameter). When the scaling term is estimated based on the data, the test statistic—under certain conditions—follows a Student's ''t'' distribution. The ''t''-test's most common application is to test whether the means of two populations are different. History The term "''t''-statistic" is abbreviated from "hypothesis test statistic". In statistics, the t-distribution was first derived as a Posterior probability, posterior distribution in 1876 by Friedrich Robert Helmert, Helmert and Jacob Lüroth, Lüroth. The t-distribution also appeared in a more general form as Pearson Type Pearson distribution, IV di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ANOVA
Analysis of variance (ANOVA) is a collection of statistical models and their associated estimation procedures (such as the "variation" among and between groups) used to analyze the differences among means. ANOVA was developed by the statistician Ronald Fisher. ANOVA is based on the law of total variance, where the observed variance in a particular variable is partitioned into components attributable to different sources of variation. In its simplest form, ANOVA provides a statistical test of whether two or more population means are equal, and therefore generalizes the ''t''-test beyond two means. In other words, the ANOVA is used to test the difference between two or more means. History While the analysis of variance reached fruition in the 20th century, antecedents extend centuries into the past according to Stigler. These include hypothesis testing, the partitioning of sums of squares, experimental techniques and the additive model. Laplace was performing hypothesis testing i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rubin Causal Model
The Rubin causal model (RCM), also known as the Neyman–Rubin causal model, is an approach to the statistical analysis of cause and effect based on the framework of potential outcomes, named after Donald Rubin. The name "Rubin causal model" was first coined by Paul W. Holland. The potential outcomes framework was first proposed by Jerzy Neyman in his 1923 Master's thesis,Neyman, Jerzy. ''Sur les applications de la theorie des probabilites aux experiences agricoles: Essai des principes.'' Master's Thesis (1923). Excerpts reprinted in English, Statistical Science, Vol. 5, pp. 463–472. ( D. M. Dabrowska, and T. P. Speed, Translators.) though he discussed it only in the context of completely randomized experiments. Rubin extended it into a general framework for thinking about causation in both observational and experimental studies. Introduction The Rubin causal model is based on the idea of potential outcomes. For example, a person would have a particular income at age 4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Statistical Methods For Research Workers
''Statistical Methods for Research Workers'' is a classic book on statistics, written by the statistician R. A. Fisher. It is considered by some to be one of the 20th century's most influential books on statistical methods, together with his ''The Design of Experiments'' (1935). It was originally published in 1925, by Oliver & Boyd (Edinburgh); the final and posthumous 14th edition was published in 1970. Reviews According to Denis Conniffe: Ronald A. Fisher was "interested in application and in the popularization of statistical methods and his early book ''Statistical Methods for Research Workers'', published in 1925, went through many editions and motivated and influenced the practical use of statistics in many fields of study. His ''Design of Experiments'' (1935) romotedstatistical technique and application. In that book he emphasized examples and how to design experiments systematically from a statistical point of view. The mathematical justification of the methods ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isis (journal)
''Isis'' is a quarterly peer-reviewed academic journal published by the University of Chicago Press. It covers the history of science, history of medicine, and the history of technology, as well as their cultural influences. It contains original research articles and extensive book reviews and review essays. Furthermore, sections devoted to one particular topic are published in each issue in open access. These sections consist of the Focus section, the Viewpoint section and the Second Look section. History The journal was established by George Sarton and the first issue appeared in March 1913. Contributions were originally in any of four European languages (English, French, German, and Italian), but since the 1920s, only English has been used. Publication is partly supported by an endowment from the Dibner Fund. Two associated publications are ''Osiris'' (established 1936 by Sarton) and the ''Isis Current Bibliography''. The publication of the journal was interrupted in 1914 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Joseph Jastrow
Joseph Jastrow (January 30, 1863 – January 8, 1944) was a Polish-born American psychologist, noted for inventions in experimental psychology, design of experiments, and psychophysics. He also worked on the phenomena of optical illusions, and a number of well-known optical illusions (notably the Jastrow illusion) were either first reported in or popularized by his work. Jastrow believed that everyone had their own, often incorrect, preconceptions about psychology. One of his goals was to use the scientific method to identify truth from error, and educate the layperson, which he did through speaking tours, popular print media, and radio. Biography Jastrow was born in Warsaw, Poland. A son of Talmud scholar Marcus Jastrow, Joseph Jastrow was the younger brother of the orientalist, Morris Jastrow, Jr. Joseph Jastrow came to Philadelphia in 1866 and received his bachelor's and master's degrees from the University of Pennsylvania. During his doctoral studies at Johns Hopki ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |