|
RVSV Vaccines
Recombinant vesicular stomatitis virus vaccines (rVSV vaccines) are vaccines made using recombinant Indiana vesiculovirus. rVSV vaccines include: Vaccines *rVSV-ZEBOV vaccine against Ebola Vaccine candidates * rVSV-SUDV vaccine, a candidate against Sudan ebolavirus (development discontinued for business reasons) * rVSV-MARV vaccine, a candidate against the Marburg virus Marburg virus (MARV) is a hemorrhagic fever virus of the ''Filoviridae'' family of viruses and a member of the species '' Marburg marburgvirus'', genus ''Marburgvirus''. It causes Marburg virus disease in primates, a form of viral hemorrhagic f ... (development discontinued for business reasons) * rVSV-based vaccine candidate against Lassa fever *An HIV vaccine candida {{vaccine-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indiana Vesiculovirus
''Indiana vesiculovirus'', formerly ''Vesicular stomatitis Indiana virus'' (VSIV or VSV) is a virus in the family ''Rhabdoviridae''; the well-known ''Rabies lyssavirus'' belongs to the same family. VSIV can infect insects, cattle, horses and pigs. It has particular importance to farmers in certain regions of the world where it infects cattle. This is because its clinical presentation is identical to the very important foot and mouth disease virus. reviewed and published by WikiVet, accessed 12 October 2011. The virus is zoonotic and leads to a flu-like illness in infected humans. It is also a common laboratory virus used to study the properties of viruses in the family ''Rhabdoviridae'', as well as to study viral evolution. Properties ''Indiana vesiculovirus'' is the prototypic member of the genus ''Vesiculovirus'' of the family ''Rhabdoviridae''. VSIV is an arbovirus, and its replication occurs in the cytoplasm. Natural VSIV infections encompass two steps, cytolytic infections ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RVSV-ZEBOV Vaccine
Recombinant vesicular stomatitis virus–Zaire Ebola virus (rVSV-ZEBOV), also known as Ebola Zaire vaccine live and sold under the brand name Ervebo, is an Ebola vaccine for adults that prevents Ebola caused by the Zaire ebolavirus. When used in ring vaccination, rVSV-ZEBOV has shown a high level of protection. Around half the people given the vaccine have mild to moderate adverse effects that include headache, fatigue, and muscle pain. rVSV-ZEBOV is a recombinant, replication-competent viral vector vaccine. It consists of rice-derived recombinant human serum albumin and live attenuated recombinant vesicular stomatitis virus (VSV), which has been genetically engineered to express the main glycoprotein from the Zaire ebolavirus so as to provoke a neutralizing immune response to the Ebola virus. The vaccine was approved for medical use in the European Union and the United States in 2019. It was created by scientists at the National Microbiology Laboratory in Winnipeg, Manito ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
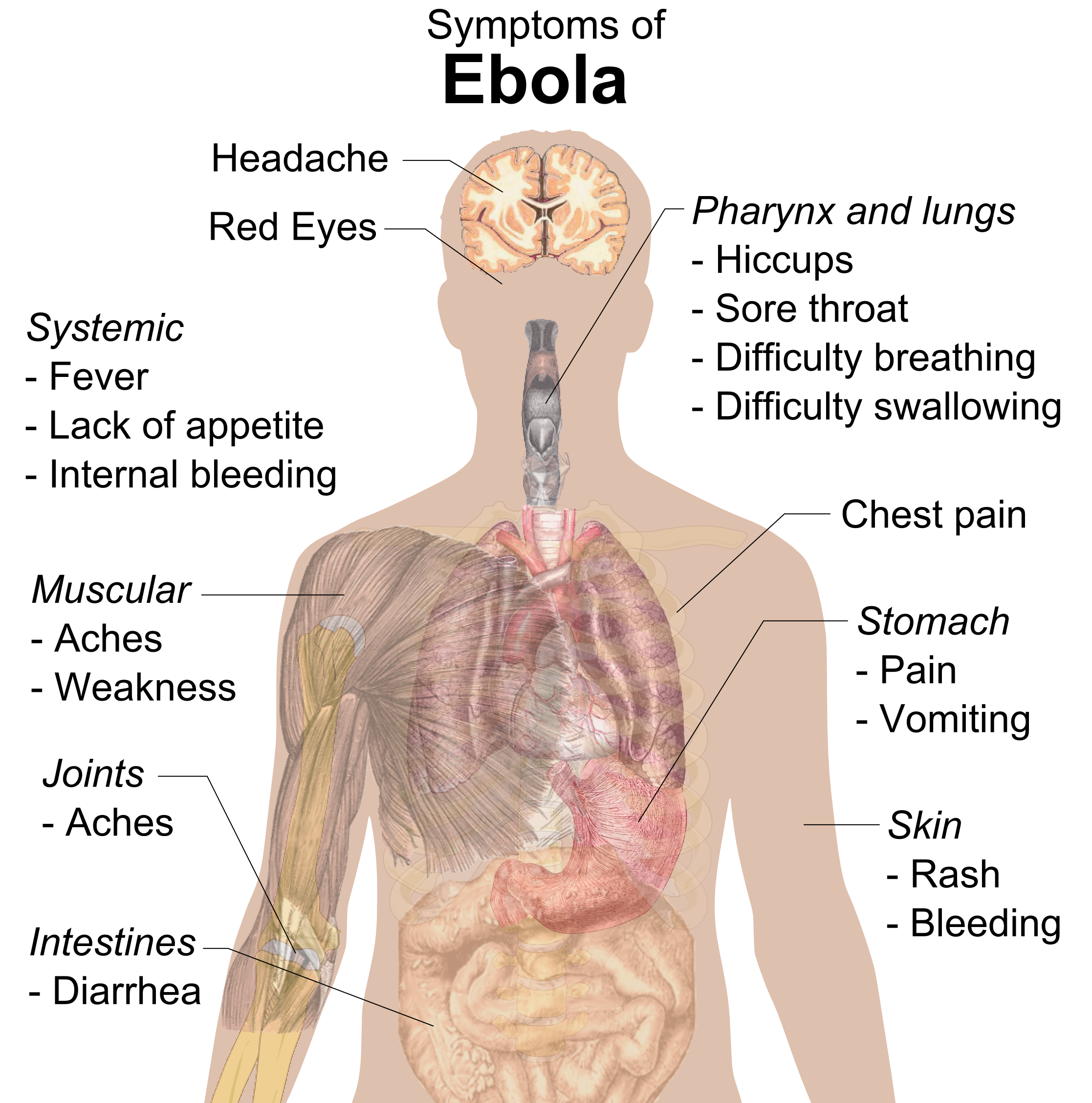
Ebola
Ebola, also known as Ebola virus disease (EVD) and Ebola hemorrhagic fever (EHF), is a viral hemorrhagic fever in humans and other primates, caused by ebolaviruses. Symptoms typically start anywhere between two days and three weeks after becoming infected with the virus. The first symptoms are usually fever, sore throat, muscle pain, and headaches. These are usually followed by vomiting, diarrhoea, rash and decreased liver and kidney function, at which point, some people begin to bleed both internally and externally. The disease kills between 25% and 90% of those infected – about 50% on average. Death is often due to shock from fluid loss, and typically occurs between six and 16 days after the first symptoms appear. Early treatment of symptoms increases the survival rate considerably compared to late start. The virus spreads through direct contact with body fluids, such as blood from infected humans or other animals, or from contact with items that have recently been conta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RVSV-SUDV Vaccine
The species ''Sudan ebolavirus'' is a virological taxon included in the genus ''Ebolavirus'', family ''Filoviridae'', order '' Mononegavirales''. The species has a single virus member, Sudan virus (SUDV). The members of the species are called Sudan ebolaviruses. It was discovered in 1977 and causes Ebola clinically indistinguishable from the ebola Zaire strain, but is less transmissible than it. Unlike with ebola Zaire there is no vaccine available. Nomenclature The name ''Sudan ebolavirus'' is derived from ''Sudan'' (the country in which Sudan virus was first discovered) and the taxonomic suffix ''ebolavirus'' (which denotes an ebolavirus species). The species was introduced in 1998 as ''Sudan Ebola virus''. In 2002, the name was changed to ''Sudan ebolavirus''. A virus of the genus ''Ebolavirus'' is a member of the species ''Sudan ebolavirus'' if: * it is endemic in Sudan and/or Uganda * it has a genome with three gene overlaps (''VP35''/''VP40'', ''GP''/''VP30'', ''VP24' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sudan Ebolavirus
The species ''Sudan ebolavirus'' is a virological taxon included in the genus ''Ebolavirus'', family ''Filoviridae'', order ''Mononegavirales''. The species has a single virus member, Sudan virus (SUDV). The members of the species are called Sudan ebolaviruses. It was discovered in 1977 and causes Ebola clinically indistinguishable from the ebola Zaire strain, but is less transmissible than it. Unlike with ebola Zaire there is no vaccine available. Nomenclature The name ''Sudan ebolavirus'' is derived from '' Sudan'' (the country in which Sudan virus was first discovered) and the taxonomic suffix ''ebolavirus'' (which denotes an ebolavirus species). The species was introduced in 1998 as ''Sudan Ebola virus''. In 2002, the name was changed to ''Sudan ebolavirus''. A virus of the genus ''Ebolavirus'' is a member of the species ''Sudan ebolavirus'' if: * it is endemic in Sudan and/or Uganda * it has a genome with three gene overlaps (''VP35''/''VP40'', ''GP''/''VP30'', ''VP ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RVSV-ZEBOV Vaccine
Recombinant vesicular stomatitis virus–Zaire Ebola virus (rVSV-ZEBOV), also known as Ebola Zaire vaccine live and sold under the brand name Ervebo, is an Ebola vaccine for adults that prevents Ebola caused by the Zaire ebolavirus. When used in ring vaccination, rVSV-ZEBOV has shown a high level of protection. Around half the people given the vaccine have mild to moderate adverse effects that include headache, fatigue, and muscle pain. rVSV-ZEBOV is a recombinant, replication-competent viral vector vaccine. It consists of rice-derived recombinant human serum albumin and live attenuated recombinant vesicular stomatitis virus (VSV), which has been genetically engineered to express the main glycoprotein from the Zaire ebolavirus so as to provoke a neutralizing immune response to the Ebola virus. The vaccine was approved for medical use in the European Union and the United States in 2019. It was created by scientists at the National Microbiology Laboratory in Winnipeg, Manito ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RVSV-MARV Vaccine
Marburg virus (MARV) is a hemorrhagic fever virus of the ''Filoviridae'' family of viruses and a member of the species ''Marburg marburgvirus'', genus ''Marburgvirus''. It causes Marburg virus disease in primates, a form of viral hemorrhagic fever. The virus is considered to be extremely dangerous. The World Health Organization (WHO) rates it as a Risk Group 4 Pathogen (requiring biosafety level 4-equivalent containment). In the United States, the National Institute of Allergy and Infectious Diseases ranks it as a Category A Priority Pathogen and the Centers for Disease Control and Prevention lists it as a Category A Bioterrorism Agent. It is also listed as a biological agent for export control by the Australia Group. The virus can be transmitted by exposure to one species of fruit bats or it can be transmitted between people via body fluids through unprotected sex and broken skin. The disease can cause haemorrhage, fever, and other symptoms similar to Ebola, which belong ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marburg Virus
Marburg virus (MARV) is a hemorrhagic fever virus of the ''Filoviridae'' family of viruses and a member of the species '' Marburg marburgvirus'', genus ''Marburgvirus''. It causes Marburg virus disease in primates, a form of viral hemorrhagic fever. The virus is considered to be extremely dangerous. The World Health Organization (WHO) rates it as a Risk Group 4 Pathogen (requiring biosafety level 4-equivalent containment). In the United States, the National Institute of Allergy and Infectious Diseases ranks it as a Category A Priority Pathogen and the Centers for Disease Control and Prevention lists it as a Category A Bioterrorism Agent. It is also listed as a biological agent for export control by the Australia Group. The virus can be transmitted by exposure to one species of fruit bats or it can be transmitted between people via body fluids through unprotected sex and broken skin. The disease can cause haemorrhage, fever, and other symptoms similar to Ebola, which belongs t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lassa Fever
Lassa fever, also known as Lassa hemorrhagic fever (LHF), is a type of viral hemorrhagic fever caused by the Lassa virus. Many of those infected by the virus do not develop symptoms. When symptoms occur they typically include fever, weakness, headaches, vomiting, and muscle pains. Less commonly there may be bleeding from the mouth or gastrointestinal tract. The risk of death once infected is about one percent and frequently occurs within two weeks of the onset of symptoms. Of those who survive, about a quarter have hearing loss, which improves within three months in about half of these cases. The disease is usually initially spread to people via contact with the urine or feces of an infected multimammate mouse. Spread can then occur via direct contact between people. Diagnosis based on symptoms is difficult. Confirmation is by laboratory testing to detect the virus's RNA, antibodies for the virus, or the virus itself in cell culture. Other conditions that may present similar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |