|
Rubidium Peroxide
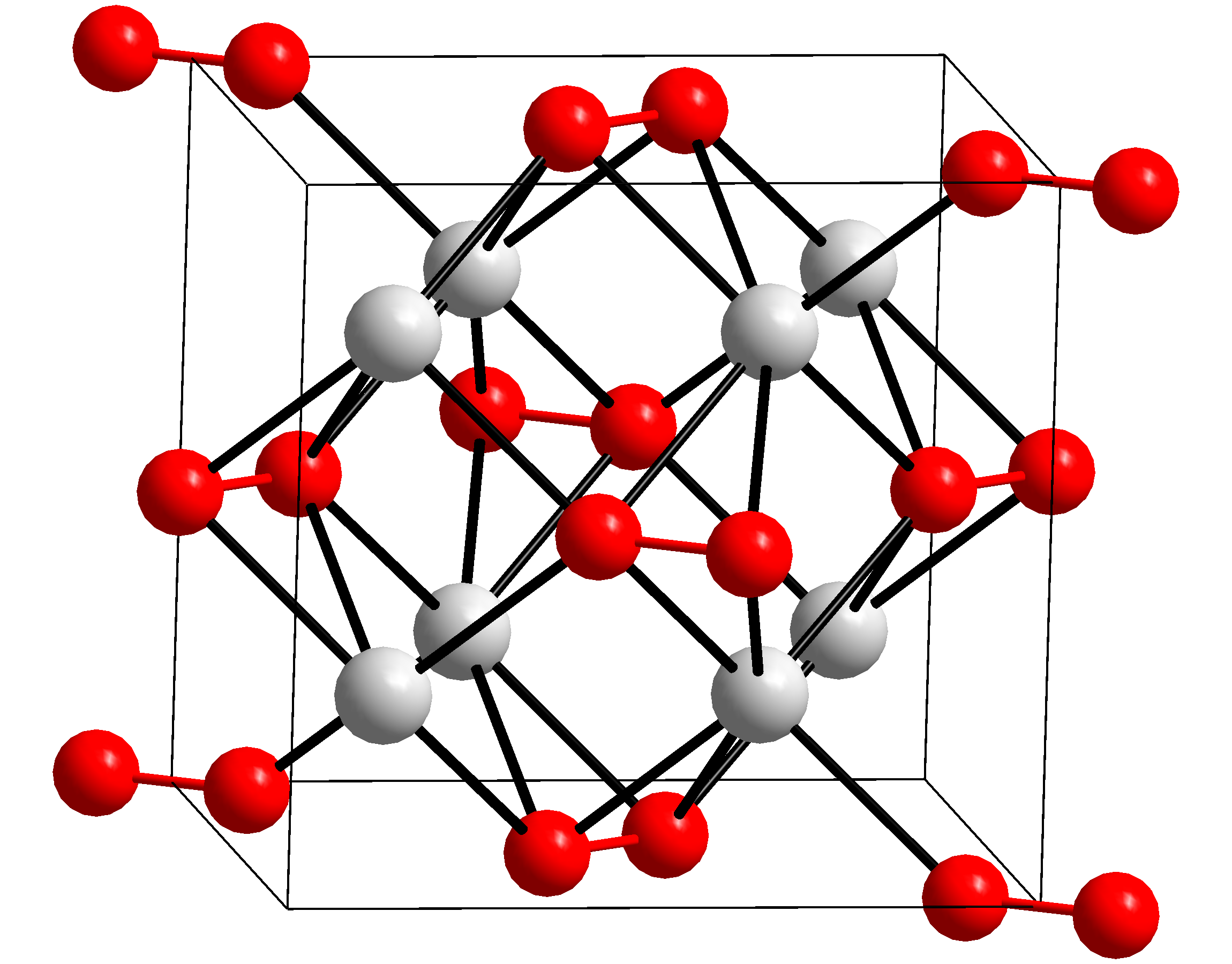
Rubidium peroxide is rubidium's peroxide with the chemical formula Rb2O2. Production Rubidium peroxide can be produced by rapidly oxidizing rubidium in liquid ammonia at −50°C. Georg Brauer (Hrsg.), unter Mitarbeit von Marianne Baudler u. a.: ''Handbuch der Präparativen Anorganischen Chemie.'' 3., umgearbeitete Auflage. Band II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3, S. 955. ::\mathrm It can also produced by pyrolysis of rubidium superoxide in vacuum. ::\mathrm Properties Rubidium peroxide is a colourless to light yellow solid with the orthorhombic crystal structure In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a .... References {{Rubidium compounds Rubidium compounds Peroxides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rubidium Oxide
Rubidium oxide is the chemical compound with the formula . Rubidium oxide is highly reactive towards water, and therefore it would not be expected to occur naturally. The rubidium content in minerals is often calculated and quoted in terms of . In reality, the rubidium is typically present as a component of (actually, an impurity in) silicate or aluminosilicate. A major source of rubidium is lepidolite, , wherein Rb sometimes replaces K. is a yellow colored solid. The related species , and are colorless, pale-yellow, and orange, respectively. The alkali metal oxides crystallise in the antifluorite structure. In the antifluorite motif the positions of the anions and cations are reversed relative to their positions in CaF2, with rubidium ions 4-coordinate (tetrahedral) and oxide ions 8-coordinate (cubic). Properties Like other alkali metal oxides, Rb2O is a strong base. Thus, Rb2O reacts exothermically with water to form rubidium hydroxide. :Rb2O + H2O → 2 RbOH So re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rubidium Superoxide
Rubidium superoxide or Rubidium hyperoxide is a compound with the formula . In terms of oxidation states, the negatively charged superoxide and positively charged rubidium give it a structural formula of (Rb+)(O2−). Chemistry It can be created by slowly exposing elemental rubidium to oxygen gas: :Rb(s) + O2(g) → RbO2(s) Like other alkali metal hyperoxides, crystals can also be grown in liquid ammonia. Between 280 and 360 °C, Rubidium superoxide will decompose, leaving not rubidium sesquioxide (Rb2O3), but rather rubidium peroxide (Rb2O2). :RbO2 (s) → 1/2 R2O2(s) + 1/2 O2(g) An even more oxygen rich compound, that of rubidium ozonide (RbO3) can be created using RbO2. Properties Roughly speaking, RbO2 has a crystal structure similar to tetragonal calcium carbide, but is rather distorted due to the Jahn–Teller effect, which makes the crystal structure less symmetrical. RbO2 is stable in dry air, but is extremely hygroscopic. The compound has been studied as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Peroxide
Lithium peroxide is the inorganic compound with the formula Li2 O2. It is a white, nonhygroscopic solid. Because of its high oxygen:mass and oxygen:volume ratios, the solid has been used to remove CO2 from the atmosphere in spacecraft. Preparation It is prepared by the reaction of hydrogen peroxide and lithium hydroxide. This reaction initially produces lithium hydroperoxide:E. Dönges "Lithium and Sodium Peroxides" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 979. :LiOH + H2O2 → LiOOH + H2O This lithium hydroperoxide has also been described as lithium peroxide monoperoxohydrate trihydrate (Li2O2·H2O2·3H2O). Dehydration of this material gives the anhydrous peroxide salt: :2 LiOOH → Li2O2 + H2O2 Li2O2 decomposes at about 450 °C to give lithium oxide: :2 Li2O2 → 2 Li2O + O2 The structure of solid Li2O2 has been determined by X-ray crystallography and density functional theory. The solid features a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Peroxide
Sodium peroxide is an inorganic compound with the formula Na2O2. This yellowish solid is the product of sodium ignited in excess oxygen. It is a strong base. This metal peroxide exists in several hydrates and peroxyhydrates including Na2O2·2H2O2·4H2O, Na2O2·2H2O, Na2O2·2H2O2, and Na2O2·8H2O.Harald Jakob, Stefan Leininger, Thomas Lehmann, Sylvia Jacobi, Sven Gutewort. "Peroxo Compounds, Inorganic". ''Ullmann's Encyclopedia of Industrial Chemistry'', 2007, Wiley-VCH, Weinheim. . The octahydrate, which is simple to prepare, is white, in contrast to the anhydrous material. Properties Sodium peroxide crystallizes with hexagonal symmetry. Upon heating, the hexagonal form undergoes a transition into a phase of unknown symmetry at 512 °C. With further heating above the 657 °C boiling point, the compound decomposes to Na2O, releasing O2.Lewis, R. J. ''Sax's Dangerous Properties of Industrial Materials, 10th ed.'', John Wiley & Sons, Inc.: 2000. : 2 Na2O2 → 2 Na2O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Peroxide
Potassium peroxide is an inorganic compound with the molecular formula K2O2. It is formed as potassium reacts with oxygen in the air, along with potassium oxide (K2O) and potassium superoxide (KO2). Potassium peroxide reacts with water to form potassium hydroxide and oxygen: : 2K2O2 + 2H2O -> 4KOH + O2 (^) Properties Potassium peroxide is a highly reactive, oxidizing white to yellowish solid which, while not flammable itself, reacts violently with flammable materials. It decomposes violently on contact with water. The standard enthalpy of formation of potassium peroxide is ΔH f 0 = −496 kJ/mol. Usage Potassium Peroxide is used as an oxidizing agent and bleach (due to the peroxide In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable. The most common peroxide is hydrogen p ...), and to purify air. References Pero ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium Peroxide
Caesium peroxide or cesium peroxide is a compound of caesium and oxygen. It can be formed from caesium metal by adding a stoichiometric amount in ammonia solution, or oxidizing the solid metal directly. :2Cs + O2 → Cs2O2 It can also be formed by the thermal decomposition of caesium superoxide: :2CsO2 → Cs2O2 + O2 Upon heating until 650°C, the compound will decompose to caesium monoxide and atomic oxygen: :\mathsf Caesium peroxide shows a Raman vibration at 743 cm−1, due to the presence of the peroxide ions. The compound is often used as a coating for photocathodes, due to its low work function In solid-state physics, the work function (sometimes spelt workfunction) is the minimum thermodynamic work (i.e., energy) needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately" m .... References Peroxides Caesium compounds {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rubidium
Rubidium is the chemical element with the symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have a density higher than water. On Earth, natural rubidium comprises two isotopes: 72% is a stable isotope 85Rb, and 28% is slightly radioactive 87Rb, with a half-life of 48.8 billion years—more than three times as long as the estimated age of the universe. German chemists Robert Bunsen and Gustav Kirchhoff discovered rubidium in 1861 by the newly developed technique, flame spectroscopy. The name comes from the Latin word , meaning deep red, the color of its emission spectrum. Rubidium's compounds have various chemical and electronic applications. Rubidium metal is easily vaporized and has a convenient spectral absorption range, making it a frequent target for laser manipulation of atoms. Rubidium is not a known nutrient for any living organisms. However, r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxide
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable. The most common peroxide is hydrogen peroxide (), colloquially known simply as "peroxide". It is marketed as solutions in water at various concentrations. Many organic peroxides are known as well. In addition to hydrogen peroxide, some other major classes of peroxides are: * Peroxy acids, the peroxy derivatives of many familiar acids, examples being peroxymonosulfuric acid and peracetic acid, and their salts, one example of which is potassium peroxydisulfate. * Main group peroxides, compounds with the linkage (E = main group element). * Metal peroxides, examples being barium peroxide (), sodium peroxide () and zinc peroxide Zinc peroxide (ZnO2) appears as a bright yellow powder at room temperature. It was historically used as a surgical antiseptic. More recently zinc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is both causti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Georg Brauer
Georg Karl Brauer (born 11. April 1908 in Bochum, died 26. February 2001 in Freiburg im Breisgau) was a German chemist. Life Brauer was the son of the chemist Eberhard Brauer and Elisabeth Brauer, a daughter of Wilhelm Ostwald. From 1926 to 1932, Brauer studied in Leipzig and Freiburg. He received his doctorate under supervision of Eduard Zintl Eduard Zintl (21 January 1898 – 17 January 1941) was a German chemist. He gained prominence for research on intermetallic compounds. Family background After his family moved from Weiden and Bayreuth to Munich and after he had finished school ... in Freiburg in 1933. In 1941, he received is habilitation at the TH Darmstadt. In 1946, he became an extraordinary professor in Freiburg. From 1959 to 1976, he was a full professor. Starting in 1976, he was a emeritus professor. Research Brauer's research included the chemistry and crystal chemistry of intermetallic compounds and alloys. He investigated binary systems of transition metal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marianne Baudler
Marianne Baudler (27 April 1921 – 5 March 2003) was a German chemist. She is known for her research on phosphorus. Life Marianne Baudler was born in Stettin. She started studying Chemistry at the TH Dresden in April 1940 and finished her studies with a Diplom in 1943. From 1943 to 1946, she worked on her dissertation in the group of Franz Fehér at the University of Göttingen. Starting in 1949, Baudler performed research at the University of Cologne. In 1952, she finished her habilitation. In 1963 she became extraordinary professor at the University of Cologne. In 1968, the full professorship followed. From 1986 on, she was an emeritus professor. Research Her research focused on: * Small-ring phosphorus compounds * Phosphorus hydrides * Polycyclic organophosphanes * Mono- and polycyclic compounds of arsenic Selected publications Awards * Alfred Stork Memorial Prize in 1986 * Member of the Academy of Sciences Leopoldina starting in 1982 * Member of the Göttingen Acade ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''pyro'' "fire", "heat", "fever" and '' lysis'' "separating". Pyrolysis is most commonly used in the treatment of organic materials. It is one of the processes involved in charring wood.''Burning of wood'' , InnoFireWood's website. Accessed on 2010-02-06. In general, pyrolysis of organic substances produces volatile products and leaves , a carbon-rich solid residue. Extreme pyrolysis, which leaves mostly |

-3D-balls.png)