|
Rociletinib
Rociletinib is a medication developed to treat non-small cell lung carcinomas with a specific mutation. It is a third-generation epidermal growth factor receptor tyrosine kinase inhibitor. It was being developed by Clovis Oncology as a potential treatment for non-small-cell lung cancer. In May 2016, development of rociletinib was halted, along with its associated clinical trials, and Clovis Oncology withdrew its marketing authorisation application from the European Medicines Agency The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of medicinal products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or Euro .... References Acrylamides Experimental cancer drugs Trifluoromethyl compounds Receptor tyrosine kinase inhibitors Abandoned drugs {{antineoplastic-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clovis Oncology
Clovis Oncology is an American pharmaceutical company which mainly markets products for treatment in oncology. Clovis was founded in 2009 and is headquartered in Boulder, Colorado. The company is a publicly traded company on NASDAQ under the symbol CLVS and is in the NASDAQ Biotechnology Index with several products in its product pipeline. As of December 31, 2017, the company was not profitable and had incurred losses in each year since its inception in April 2009. In December 2022, Clovis Oncology filed for Chapter 11 bankruptcy. History Clovis Oncology was founded in 2009 by Patrick Mahaffy in Boulder, Colorado. The company was named in honor of the Mahaffy cache, a collection of Clovis period stone tools dated to 11,000 BCE, discovered in the front yard Mahaffy's home in Boulder. Product development Rociletinib The company was developing rociletinib, as a treatment for non-small cell lung cancer. A phase III trial was completed in April 2016 and had it been appr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Administration
Oral administration is a route of administration where a substance is taken through the mouth. Per os abbreviated to P.O. is sometimes used as a direction for medication to be taken orally. Many medications are taken orally because they are intended to have a systemic effect, reaching different parts of the body via the bloodstream, for example. Oral administration can be easier and less painful than other routes, such as injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients willing and able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-small Cell Lung Carcinoma
Non-small-cell lung cancer (NSCLC) is any type of epithelial lung cancer other than small-cell lung carcinoma (SCLC). NSCLC accounts for about 85% of all lung cancers. As a class, NSCLCs are relatively insensitive to chemotherapy, compared to small-cell carcinoma. When possible, they are primarily treated by surgical resection with curative intent, although chemotherapy has been used increasingly both preoperatively (neoadjuvant chemotherapy) and postoperatively (adjuvant chemotherapy). Types The most common types of NSCLC are squamous-cell carcinoma, large-cell carcinoma, and adenocarcinoma, but several other types occur less frequently. A few of the less common types are pleomorphic, carcinoid tumor, salivary gland carcinoma, and unclassified carcinoma. All types can occur in unusual histologic variants and as mixed cell-type combinations. Nonsquamous-cell carcinoma almost occupies the half of NSCLC. In the tissue classification, the central type contains about one-ninth. Som ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epidermal Growth Factor Receptor
The epidermal growth factor receptor (EGFR; ErbB-1; HER1 in humans) is a transmembrane protein that is a receptor for members of the epidermal growth factor family (EGF family) of extracellular protein ligands. The epidermal growth factor receptor is a member of the ErbB family of receptors, a subfamily of four closely related receptor tyrosine kinases: EGFR (ErbB-1), HER2/neu (ErbB-2), Her 3 (ErbB-3) and Her 4 (ErbB-4). In many cancer types, mutations affecting EGFR expression or activity could result in cancer. Epidermal growth factor and its receptor was discovered by Stanley Cohen of Vanderbilt University. Cohen shared the 1986 Nobel Prize in Medicine with Rita Levi-Montalcini for their discovery of growth factors. Deficient signaling of the EGFR and other receptor tyrosine kinases in humans is associated with diseases such as Alzheimer's, while over-expression is associated with the development of a wide variety of tumors. Interruption of EGFR signalling, either by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosine Kinase Inhibitor
A tyrosine kinase inhibitor (TKI) is a pharmaceutical drug that inhibits tyrosine kinases. Tyrosine kinases are enzymes responsible for the activation of many proteins by signal transduction cascades. The proteins are activated by adding a phosphate group to the protein (phosphorylation), a step that TKIs inhibit. TKIs are typically used as anticancer drugs. For example, they have substantially improved outcomes in chronic myelogenous leukemia. They have also been used to treat other diseases, such as idiopathic pulmonary fibrosis. They are also called tyrphostins, the short name for "tyrosine phosphorylation inhibitor", originally coined in a 1988 publication, which was the first description of compounds inhibiting the catalytic activity of the epidermal growth factor receptor (EGFR). The 1988 study was the first demonstration of a systematic search and discovery of small-molecular-weight inhibitors of tyrosine phosphorylation, which do not inhibit protein kinases that phospho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Development
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process – from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials – to approved vaccine or drug typically takes more than a decade. New chemical entity development Broadly, the process of drug development can be divided into preclinical and clinical work. Pre-clinical New chemical entities (NCEs, also known as new molecular entities or NMEs) are compounds that emerge from the process of drug discovery. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-small-cell Lung Cancer
Non-small-cell lung cancer (NSCLC) is any type of epithelial lung cancer other than small-cell lung carcinoma (SCLC). NSCLC accounts for about 85% of all lung cancers. As a class, NSCLCs are relatively insensitive to chemotherapy, compared to small-cell carcinoma. When possible, they are primarily treated by surgical resection with curative intent, although chemotherapy has been used increasingly both preoperatively ( neoadjuvant chemotherapy) and postoperatively (adjuvant chemotherapy). Types The most common types of NSCLC are squamous-cell carcinoma, large-cell carcinoma, and adenocarcinoma, but several other types occur less frequently. A few of the less common types are pleomorphic, carcinoid tumor, salivary gland carcinoma, and unclassified carcinoma. All types can occur in unusual histologic variants and as mixed cell-type combinations. Nonsquamous-cell carcinoma almost occupies the half of NSCLC. In the tissue classification, the central type contains about one-ninth. So ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marketing Authorisation Application
Marketing Authorisation Application (MAA) is an application submitted by a drug manufacturer seeking marketing authorisation, that is permission to bring a medicinal product (for example, a new medicine or generic medicine) to the market. MAA is part of the official procedure before the Medicines and Healthcare products Regulatory Agency in the United Kingdom and the Committee for Medicinal Products for Human Use of the European Medicines Agency, a specialised agency of the European Commission. In the United States The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country primarily located in North America. It consists of 50 states, a federal district, five major unincorporated territori ..., the equivalent process is called New Drug Application. References {{reflist Drug safety ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of medicinal products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would not onl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acrylamides
Acrylamide (or acrylic amide) is an organic compound with the chemical formula CH2=CHC(O)NH2. It is a white odorless solid, soluble in water and several organic solvents. From the chemistry perspective, acrylamide is a vinyl-substituted primary amide (CONH2). It is produced industrially mainly as a precursor to polyacrylamides, which find many uses as water-soluble thickeners and flocculation agents. Acrylamide forms in burnt areas of food, particularly starchy foods like potatoes, when cooked with high heat, above . Acrylamide is highly toxic, linked to cancer in animal testing though not likely to be carcinogenic for humans, but its main derivative polyacrylamide is nontoxic. The possibility that this innocuous polymer contains traces of its hazardous precursor has long attracted attention. Because acrylamide is volatile and hazardous, it is mainly handled as an aqueous solution. Production Acrylamide can be prepared by the hydration of acrylonitrile: :CH2=CHCN + H2O → C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Experimental Cancer Drugs
An experiment is a procedure carried out to support or refute a hypothesis, or determine the efficacy or likelihood of something previously untried. Experiments provide insight into cause-and-effect by demonstrating what outcome occurs when a particular factor is manipulated. Experiments vary greatly in goal and scale but always rely on repeatable procedure and logical analysis of the results. There also exist natural experimental studies. A child may carry out basic experiments to understand how things fall to the ground, while teams of scientists may take years of systematic investigation to advance their understanding of a phenomenon. Experiments and other types of hands-on activities are very important to student learning in the science classroom. Experiments can raise test scores and help a student become more engaged and interested in the material they are learning, especially when used over time. Experiments can vary from personal and informal natural comparisons (e. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
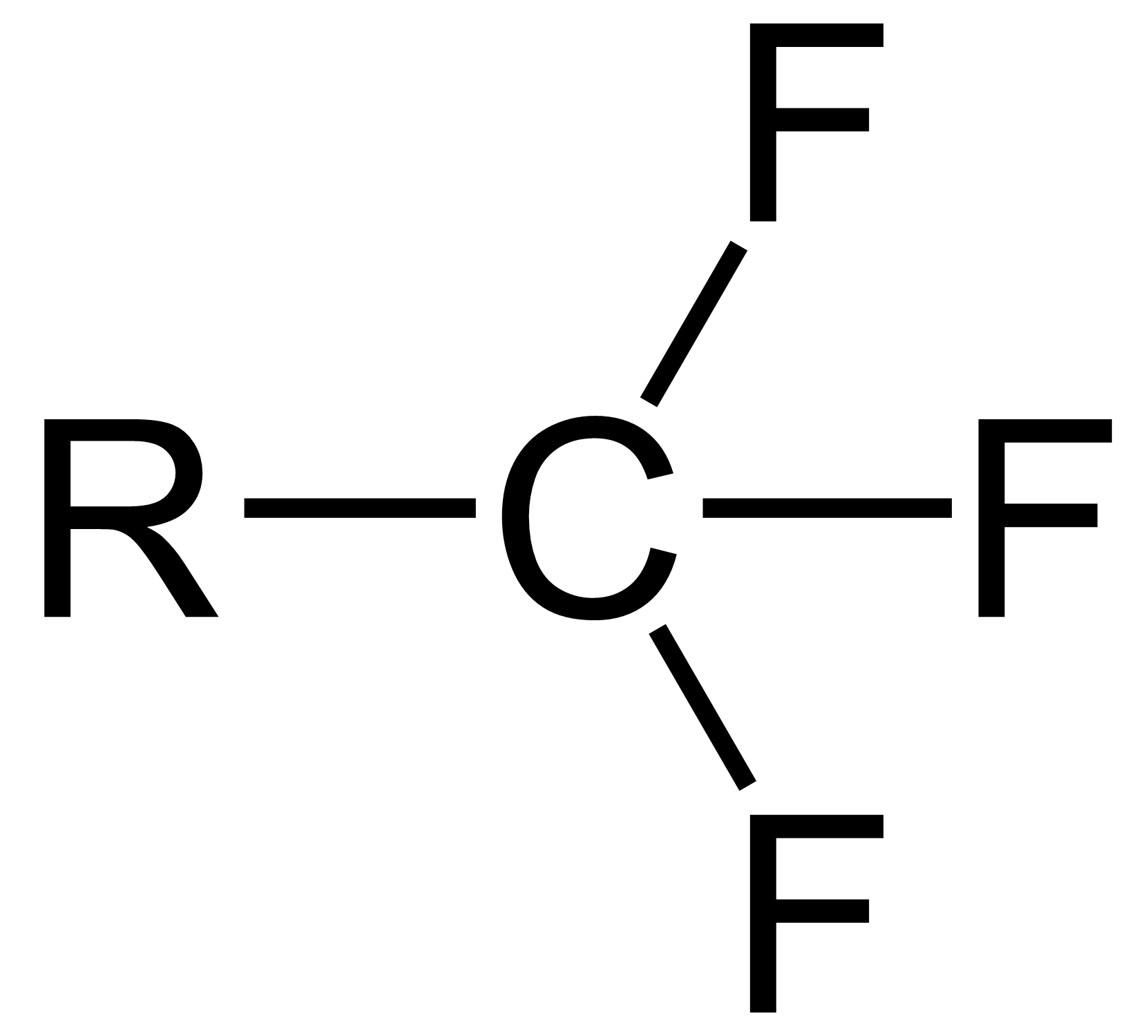
Trifluoromethyl Compounds
The trifluoromethyl group is a functional group that has the formula -CF3. The naming of is group is derived from the methyl group (which has the formula -CH3), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluoromethane H–, 1,1,1-trifluoroethane –, and hexafluoroacetone –CO–. Compounds with this group are a subclass of the organofluorines. Properties The trifluoromethyl group has a significant electronegativity that is often described as being intermediate between the electronegativities of fluorine and chlorine. For this reason, trifluoromethyl-substituted compounds are often strong acids, such as trifluoromethanesulfonic acid and trifluoroacetic acid. Conversely, the trifluoromethyl group lowers the basicity of compounds like trifluoroethanol. Uses The trifluoromethyl group occurs in certain pharmaceuticals, drugs, and abiotically synthesized natural fluorocarbon based compounds. The medicinal use of the trifloromethyl group dates from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |