|
Rhenium Hexafluoride
Rhenium hexafluoride, also rhenium(VI) fluoride, (ReF6) is a compound of rhenium and fluorine and one of the seventeen known binary hexafluorides. Synthesis Rhenium hexafluoride is made by combining rhenium heptafluoride with additional rhenium metal at 300 °C in a pressure vessel. :6 + Re → 7 Description Rhenium hexafluoride is a liquid at room temperature. At 18.5 °C, it freezes into a yellow solid. The boiling point is 33.7 °C. The solid structure measured at −140 °C is orthorhombic space group ''Pnma''. Lattice parameters are ''a'' = 9.417 Å, ''b'' = 8.570 Å, and ''c'' = 4.965 Å. There are four formula units (in this case, discrete molecules) per unit cell, giving a density of 4.94 g·cm−3. The ReF6 molecule itself (the form important for the liquid or gas phase) has octahedral molecular geometry, which has point group ('' Oh''). The Re–F bond length In molecular geometr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one of the rarest elements in the Earth's crust. Rhenium has the third-highest melting point and highest boiling point of any stable element at 5869 K. Rhenium resembles manganese and technetium chemically and is mainly obtained as a by-product of the extraction and refinement of molybdenum and copper ores. Rhenium shows in its compounds a wide variety of oxidation states ranging from −1 to +7. Discovered by Walter Noddack, Ida Tacke and Otto Berg in 1925, rhenium was the last stable element to be discovered. It was named after the river Rhine in Europe, from which the earliest samples had been obtained and worked commercially. Nickel-based superalloys of rhenium are used in combustion chambers, turbine blades, and exhaust nozzle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octahedral Molecular Geometry
In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The octahedron has eight faces, hence the prefix '' octa''. The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no bonds between the ligand atoms. A perfect octahedron belongs to the point group Oh. Examples of octahedral compounds are sulfur hexafluoride SF6 and molybdenum hexacarbonyl Mo(CO)6. The term "octahedral" is used somewhat loosely by chemists, focusing on the geometry of the bonds to the central atom and not considering differences among the ligands themselves. For example, , which is not octahedral in the mathematical sense due to the orientation of the bonds, is referred to as octahedral. The concept of octahedral coordination geometry was developed by Alfred Wer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhenium Compounds
Rhenium compounds are compounds formed by the transition metal rhenium (Re). Rhenium can form in many oxidation states, and compounds are known for every oxidation state from -3 to +7 except +2, although the oxidation states +7, +6, +4, and +2 are the most common. Rhenium is most available commercially as salts of perrhenate, including sodium and ammonium perrhenates. These are white, water-soluble compounds. Tetrathioperrhenate anion eS4sup>− is possible. Chalcogenides Oxides Rhenium(IV) oxide (or rhenium dioxide) is an oxide of rhenium, with the formula ReO2. This gray to black crystalline solid is a laboratory reagent that can be used as a catalyst. It adopts the rutile structure. It forms via comproportionation: :2 Re2O7 + 3 Re → 7 ReO2 Single crystals are obtained by chemical transport, using iodine as the transporting agent. At high temperatures it undergoes disproportionation. It forms perrhenates with alkaline hydrogen peroxide and oxidizing acids. In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CRC Handbook Of Chemistry And Physics
The ''CRC Handbook of Chemistry and Physics'' is a comprehensive one-volume reference resource for science research. First published in 1914, it is currently () in its 103rd edition, published in 2022. It is sometimes nicknamed the "Rubber Bible" or the "Rubber Book", as CRC originally stood for "Chemical Rubber Company". As late as the 1962–1963 edition (3604 pages) the ''Handbook'' contained myriad information for every branch of science and engineering. Sections in that edition include: Mathematics, Properties and Physical Constants, Chemical Tables, Properties of Matter, Heat, Hygrometric and Barometric Tables, Sound, Quantities and Units, and Miscellaneous. Earlier editions included sections such as "Antidotes of Poisons", "Rules for Naming Organic Compounds", "Surface Tension of Fused Salts", "Percent Composition of Anti-Freeze Solutions", "Spark-gap Voltages", "Greek Alphabet", "Musical Scales", "Pigments and Dyes", "Comparison of Tons and Pounds", "Twist Drill and St ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deposition (chemistry)
In chemistry, deposition occurs when molecules settle out of a solution. Deposition can be viewed as a reverse process to dissolution or particle re-entrainment. See also * Atomic layer deposition * Chemical vapor deposition * Deposition (physics) * Fouling * Physical vapor deposition * Thin-film deposition * Fused filament fabrication Fused filament fabrication (FFF), also known as fused deposition modeling (with the trademarked acronym FDM), or called ''filament freeform fabrication'', is a 3D printing process that uses a continuous filament of a thermoplastic material. Filam ... Chemical processes Physical chemistry {{Chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of the molecule. Explanation Bond length is related to bond order: when more electrons participate in bond formation the bond is shorter. Bond length is also inversely related to bond strength and the bond dissociation energy: all other factors being equal, a stronger bond will be shorter. In a bond between two identical atoms, half the bond distance is equal to the covalent radius. Bond lengths are measured in the solid phase by means of X-ray diffraction, or approximated in the gas phase by microwave spectroscopy. A bond between a given pair of atoms may vary between different molecules. For example, the carbon to hydrogen bonds in methane are different from those in methyl chloride. It is however possible to make generalizations whe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octahedral Symmetry
A regular octahedron has 24 rotational (or orientation-preserving) symmetries, and 48 symmetries altogether. These include transformations that combine a reflection and a rotation. A cube has the same set of symmetries, since it is the polyhedron that is dual to an octahedron. The group of orientation-preserving symmetries is ''S''4, the symmetric group or the group of permutations of four objects, since there is exactly one such symmetry for each permutation of the four diagonals of the cube. Details Chiral and full (or achiral) octahedral symmetry are the discrete point symmetries (or equivalently, symmetries on the sphere) with the largest symmetry groups compatible with translational symmetry. They are among the crystallographic point groups of the cubic crystal system. As the hyperoctahedral group of dimension 3 the full octahedral group is the wreath product S_2 \wr S_3 \simeq S_2^3 \rtimes S_3,and a natural way to identify its elements is as pairs (m, n) with m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
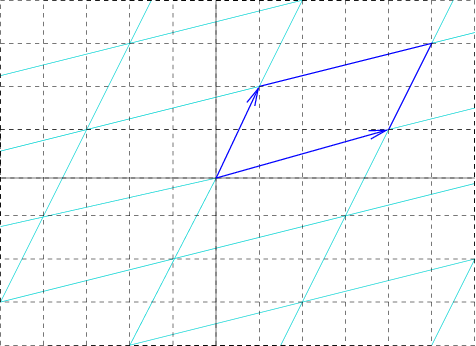
Unit Cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessarily have unit size, or even a particular size at all. Rather, the primitive cell is the closest analogy to a unit vector, since it has a determined size for a given lattice and is the basic building block from which larger cells are constructed. The concept is used particularly in describing crystal structure in two and three dimensions, though it makes sense in all dimensions. A lattice can be characterized by the geometry of its unit cell, which is a section of the tiling (a parallelogram or parallelepiped) that generates the whole tiling using only translations. There are two special cases of the unit cell: the primitive cell and the conventional cell. The primitive cell is a unit cell corresponding to a single lattice point, it is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases. Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning 'flow' gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist Henri Moissan isolate elemental fluorine using low-temperature electrolysis, a process still employed for modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formula Unit
In chemistry, a formula unit is the empirical formula of any ionic or covalent network solid compound used as an independent entity for stoichiometric calculations. It is the lowest whole number ratio of ions represented in an ionic compound. Examples include ionic and and covalent networks such as and C (as diamond or graphite).Steven S. Zumdahl; Susan A. Zumdahl (2000), ''Chemistry'' (5 ed.), Houghton Mifflin, pp. 470-6, Ionic compounds do not exist as individual molecules; a formula unit thus indicates the lowest reduced ratio of ions in the compound. In mineralogy, as minerals are almost exclusively either ionic or network solids, the formula unit is used. The number of formula units (Z) and the dimensions of the crystallographic axes are used in defining the unit cell In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |