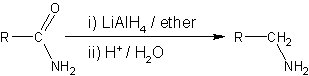|
ReO3
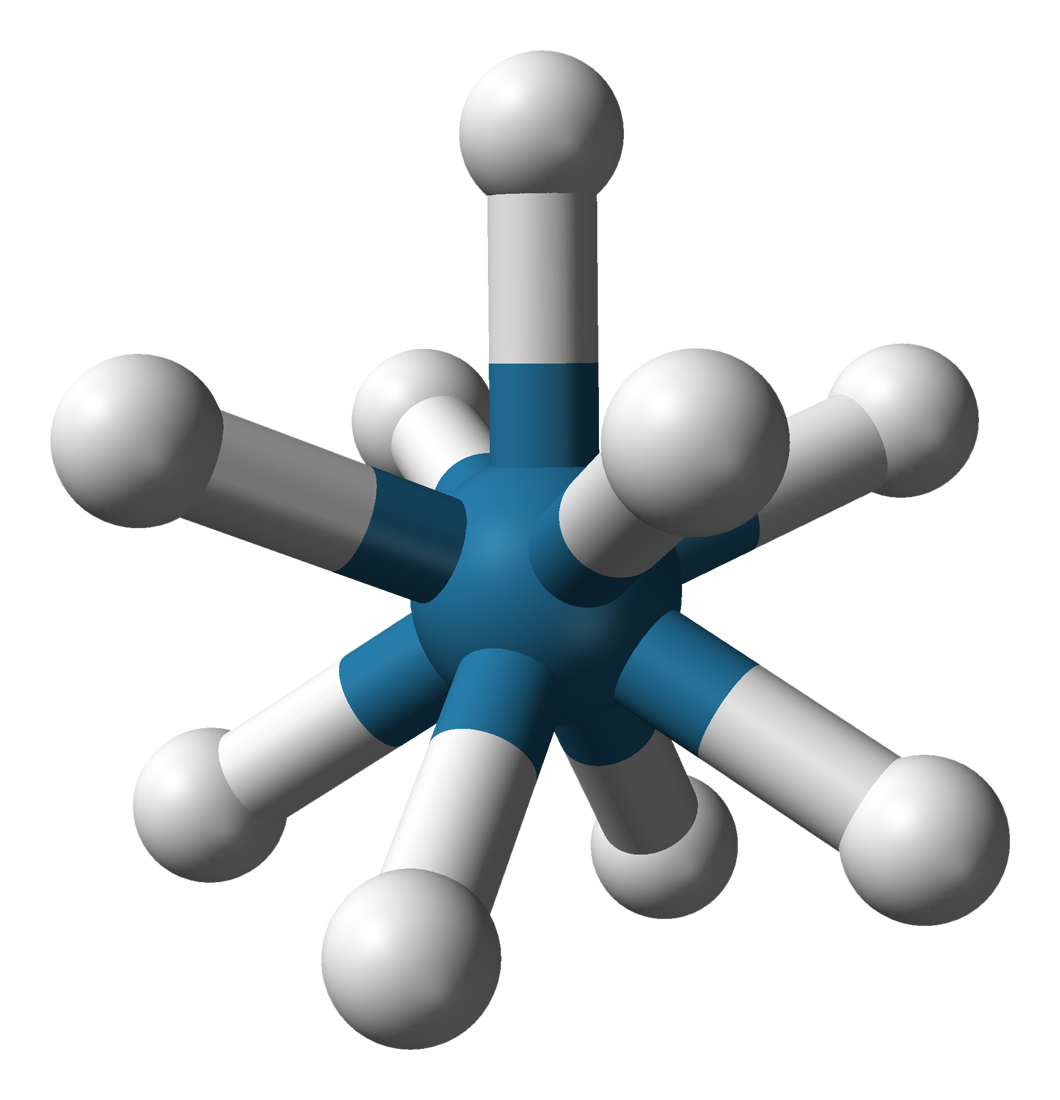
Rhenium trioxide or rhenium(VI) oxide is an inorganic compound with the formula ReO3. It is a red solid with a metallic lustre that resembles copper in appearance. It is the only stable trioxide of the Group 7 elements ( Mn, Tc, Re). Preparation and structure Rhenium trioxide can be formed by reducing rhenium(VII) oxide with carbon monoxide at 200 °C or elemental rhenium at 400 °C. :Re2O7 + CO → 2 ReO3 + CO2 :3 Re2O7 + Re → 7 ReO3 Re2O7 can also be reduced with dioxane. Rhenium trioxide crystallizes with a primitive cubic unit cell, with a lattice parameter of 3.742 Å (374.2 pm). The structure of ReO3 is similar to that of perovskite (ABO3), without the large A cation at the centre of the unit cell. Each rhenium center is surrounded by an octahedron defined by six oxygen centers. These octahedra share corners to form the 3-dimensional structure. The coordination number of O is 2, because each oxygen atom has 2 neighbouring Re atoms., p. 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhenium Trioxide Polyhedra
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one of the rarest elements in the Earth's crust. Rhenium has the third-highest melting point and highest boiling point of any stable element at 5869 K. Rhenium resembles manganese and technetium chemically and is mainly obtained as a by-product of the extraction and refinement of molybdenum and copper ores. Rhenium shows in its compounds a wide variety of oxidation states ranging from −1 to +7. Discovered by Walter Noddack, Ida Tacke and Otto Berg in 1925, rhenium was the last stable element to be discovered. It was named after the river Rhine in Europe, from which the earliest samples had been obtained and worked commercially. Nickel-based superalloys of rhenium are used in combustion chambers, turbine blades, and exhaus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perrhenic Acid
Perrhenic acid is the chemical compound with the formula . It is obtained by evaporating aqueous solutions of . Conventionally, perrhenic acid is considered to have the formula , and a species of this formula forms when rhenium(VII) oxide sublimes in the presence of water or steam. When a solution of is kept for a period of months, it breaks down and crystals of are formed, which contain tetrahedral . For most purposes, perrhenic acid and rhenium(VII) oxide are used interchangeably. Rhenium can be dissolved in nitric or concentrated sulfuric acid to produce perrhenic acid. Properties The structure of solid perrhenic acid is . This species is a rare example of a metal oxide coordinated to water; most often metal–oxo–aquo species are unstable with respect to their corresponding hydroxides: : The two rhenium atoms have different bonding geometries, with one being tetrahedral and the other octahedral, and with the water ligands coordinated to the latter. Gaseous perrhenic ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one of the rarest elements in the Earth's crust. Rhenium has the third-highest melting point and highest boiling point of any stable element at 5869 K. Rhenium resembles manganese and technetium chemically and is mainly obtained as a by-product of the extraction and refinement of molybdenum and copper ores. Rhenium shows in its compounds a wide variety of oxidation states ranging from −1 to +7. Discovered by Walter Noddack, Ida Tacke and Otto Berg in 1925, rhenium was the last stable element to be discovered. It was named after the river Rhine in Europe, from which the earliest samples had been obtained and worked commercially. Nickel-based superalloys of rhenium are used in combustion chambers, turbine blades, and exhaust nozzl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pearson Symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure, and was originated by W. B. Pearson. The symbol is made up of two letters followed by a number. For example: * Diamond structure, ''cF''8 * Rutile structure, ''tP''6 The two (italicised) letters specify the Bravais lattice. The lower-case letter specifies the crystal family, and the upper-case letter the centering type. The number at the end of the Pearson symbol gives the number of the atoms in the conventional unit cell.Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005 IR-3.4.4, pp. 49–51; IR-11.5, pp. 241–242. |
Perovskite (structure)
A perovskite is any material with a crystal structure following the formula ABX3, which was first discovered as the Perovskite, mineral called perovskite, which consists of calcium titanium oxide (CaTiO3). The mineral was first discovered in the Ural Mountains, Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). 'A' and 'B' are two positively charged ions (i.e. cations), often of very different sizes, and X is a negatively charged ion (an anion, frequently oxide) that bonds to both cations. The 'A' atoms are generally larger than the 'B' atoms. The ideal Cubic crystal system, cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedron, cuboctahedral coordination. Additional perovskite forms may exist where either/both the A and B sites have a configuration of A1x-1A2x and/or B1y-1B2y and the X may deviate from the ideal coordination configu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhenium Compounds
Rhenium compounds are compounds formed by the transition metal rhenium (Re). Rhenium can form in many oxidation states, and compounds are known for every oxidation state from -3 to +7 except +2, although the oxidation states +7, +6, +4, and +2 are the most common. Rhenium is most available commercially as salts of perrhenate, including sodium and ammonium perrhenates. These are white, water-soluble compounds. Tetrathioperrhenate anion eS4sup>− is possible. Chalcogenides Oxides Rhenium(IV) oxide (or rhenium dioxide) is an oxide of rhenium, with the formula ReO2. This gray to black crystalline solid is a laboratory reagent that can be used as a catalyst. It adopts the rutile structure. It forms via comproportionation: :2 Re2O7 + 3 Re → 7 ReO2 Single crystals are obtained by chemical transport, using iodine as the transporting agent. At high temperatures it undergoes disproportionation. It forms perrhenates with alkaline hydrogen peroxide and oxidizing acids. In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amide Reduction
Amide reduction is a reaction in organic synthesis where an amide is reduced to either an amine or an aldehyde functional group. Catalytic hydrogenation Catalytic hydrogenation can be used to reduce amides to amines; however, the process often requires high hydrogenation pressures and reaction temperatures to be effective (i.e. often requiring pressures above 197 atm and temperatures exceeding 200 °C). Selective catalysts for the reaction include copper chromite, rhenium trioxide and rhenium(VII) oxide or bimetallic catalyst. Non-catalytic routes to amines Reducing agents able to effect this reaction include metal hydrides such as lithium aluminium hydride, or lithium borohydride in mixed solvents of tetrahydrofuran and methanol. : Noncatalytic routes to aldehydes N,N-disubstituted amides can be reduced to aldehydes by using an excess of the amide: :R(CO)NRR' + LiAlH4 → RCHO + HNRR' With further reduction the alcohol Alcohol most commonly refers to: * Alcohol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some sta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: '' total synthesis'', '' semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. More generally, the term can be applied to any desymmetrizing reaction of the following type, regardless of whether it is a redox or some other type of process: :2A -> A' + A'' Examples * Mercury(I) chloride disproportionates upon UV-irradiation: :Hg2Cl2 → Hg + HgCl2 * Phosphorous acid disproportionates upon heating to give phosphoric acid and phosphine: :4 → 3 H3PO4 + PH3 *Desymmetrizing reactions are sometimes referred to as disproportionation, as illustrated by the thermal degradation of bicarbonate: :2 → + H2CO3 :The oxidation numbers remain constant in this acid-base reaction. This process is also called autoionization. *Another variant on disproportionation is radical disproportionation, in which two radicals form an alkene and an alkane. : R ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SI Electromagnetism Units
See also * SI * Speed of light * List of electromagnetism equations This article summarizes equations in the theory of electromagnetism. Definitions Here subscripts ''e'' and ''m'' are used to differ between electric and magnetic charges. The definitions for monopoles are of theoretical interest, although rea ... References External links History of the electrical units. Electromagnetism Lists of units of measurement {{Electromagnetism-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |