|
RTA 408
Omaveloxolone, sold under the brand name Skyclarys, is a medication used for the treatment of Friedreich's ataxia. It is taken by mouth. The most common side effects include an increase in alanine transaminase and an increase of aspartate aminotransferase, which can be signs of liver damage, headache, nausea, abdominal pain, fatigue, diarrhea and musculoskeletal pain. Omaveloxolone was approved for medical use in the United States in February 2023. The US Food and Drug Administration (FDA) considers it to be a first-in-class medication. Medical uses Omaveloxolone is indicated for the treatment of Friedreich's ataxia. Friedreich's ataxia causes progressive damage to the spinal cord, peripheral nerves, and the brain, resulting in uncoordinated muscle movement, poor balance, difficulty walking, changes in speech and swallowing, and a shortened lifespan. The condition can also cause heart disease. This disease tends to develop in children and teenagers and gradually worse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
By Mouth
Oral administration is a route of administration where a substance is taken through the mouth. Per os abbreviated to P.O. is sometimes used as a direction for medication to be taken orally. Many medications are taken orally because they are intended to have a systemic effect, reaching different parts of the body via the bloodstream, for example. Oral administration can be easier and less painful than other routes, such as injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients willing and able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reata Pharmaceuticals
Reata Pharmaceuticals, Inc. is a pharmaceutical company based in Plano, Texas. Founded in 2002, Reata is primarily focused on investigating experimental oral antioxidative and anti-inflammatory drugs, which dually activate the antioxidative transcription factor Nrf2 and inhibit the pro-inflammatory transcription factor NF-κB. Pipeline The antioxidative and anti-inflammatory compounds bardoxolone methyl and RTA 408 are the lead clinical development compounds in Reata’s portfolio. Bardoxolone Methyl Bardoxolone methyl was one of the first of the class of synthetic triterpenoids to be studied in the clinic. It has been evaluated in Phase 1 studies for cancer, Phase 2 and 3 studies for chronic kidney disease (CKD) associated with type 2 diabetes, and is currently being evaluated in a Phase 2 study for pulmonary arterial hypertension. RTA 408 Omaveloxolone (RTA 408) is a second generation member of the synthetic oleanane triterpenoid compounds and currently in clinical de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of medicinal products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would not onl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Committee For Medicinal Products For Human Use
The Committee for Medicinal Products for Human Use (CHMP), formerly known as Committee for Proprietary Medicinal Products (CPMP), is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding medicinal products for human use. See also * Committee for Medicinal Products for Veterinary Use The Committee for Medicinal Products for Veterinary Use (CVMP) is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding veterinary medicines. Text was copied from this source which is © ... References External links Committee for Medicinal Products for Human Use (CHMP) Health and the European Union {{eu-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Priority Review
Priority review is a program of the United States Food and Drug Administration (FDA) to expedite the review process for drugs that are expected to have a particularly great impact on the treatment of a disease. The priority review voucher program is a program that grants a voucher for priority review to a drug developer as an incentive to develop treatments for disease indications with limited profitability. Priority review vouchers are currently earned by pharmaceutical companies for the development and approval of drugs treating neglected tropical diseases, rare pediatric diseases, and "medical countermeasures" for terrorism. The voucher can be used for future drugs that could have wider indications for use, but the company is required to pay a fee (approximately $2.8 million) to use the voucher. When seeking approval for a drug, manufacturers can apply to the FDA for priority review. This is granted when a drug is intended to treat a serious condition and would "provide a si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fast Track (FDA)
Fast track is a designation by the United States Food and Drug Administration (FDA) of an investigational drug for expedited review to facilitate development of drugs that treat a serious or life-threatening condition and fill an unmet medical need. Fast Track designation must be requested by the drug company. The request can be initiated at any time during the drug development process. FDA will review the request and attempt to make a decision within sixty days. Purpose Fast Track is one of five Food and Drug Administration (FDA) approaches to make new drugs available as rapidly as possible: the others are priority review, breakthrough therapy, accelerated approval and Regenerative Medicine Advanced Therapy. Fast Track was introduced by the FDA Modernization Act of 1997. Requirements Fast track designation is designed to aid in the development and expedite the review of drugs which show promise in treating a serious or life-threatening disease and address an unmet medical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orphan Drug
An orphan drug is a pharmaceutical agent developed to treat medical conditions which, because they are so rare, would not be profitable to produce without government assistance. The conditions are referred to as orphan diseases. The assignment of orphan status to a disease and to drugs developed to treat it is a matter of public policy in many countries and has yielded medical breakthroughs that might not otherwise have been achieved, due to the economics of drug research and development. In the U.S. and the EU, it is easier to gain marketing approval for an orphan drug. There may be other financial incentives, such as an extended period of exclusivity, during which the producer has sole rights to market the drug. All are intended to encourage development of drugs which would otherwise lack sufficient profit motive to attract corporate research budgets and personnel. Definition According to the US Food and Drug Administration (FDA), an orphan drug is defined as one "intended for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
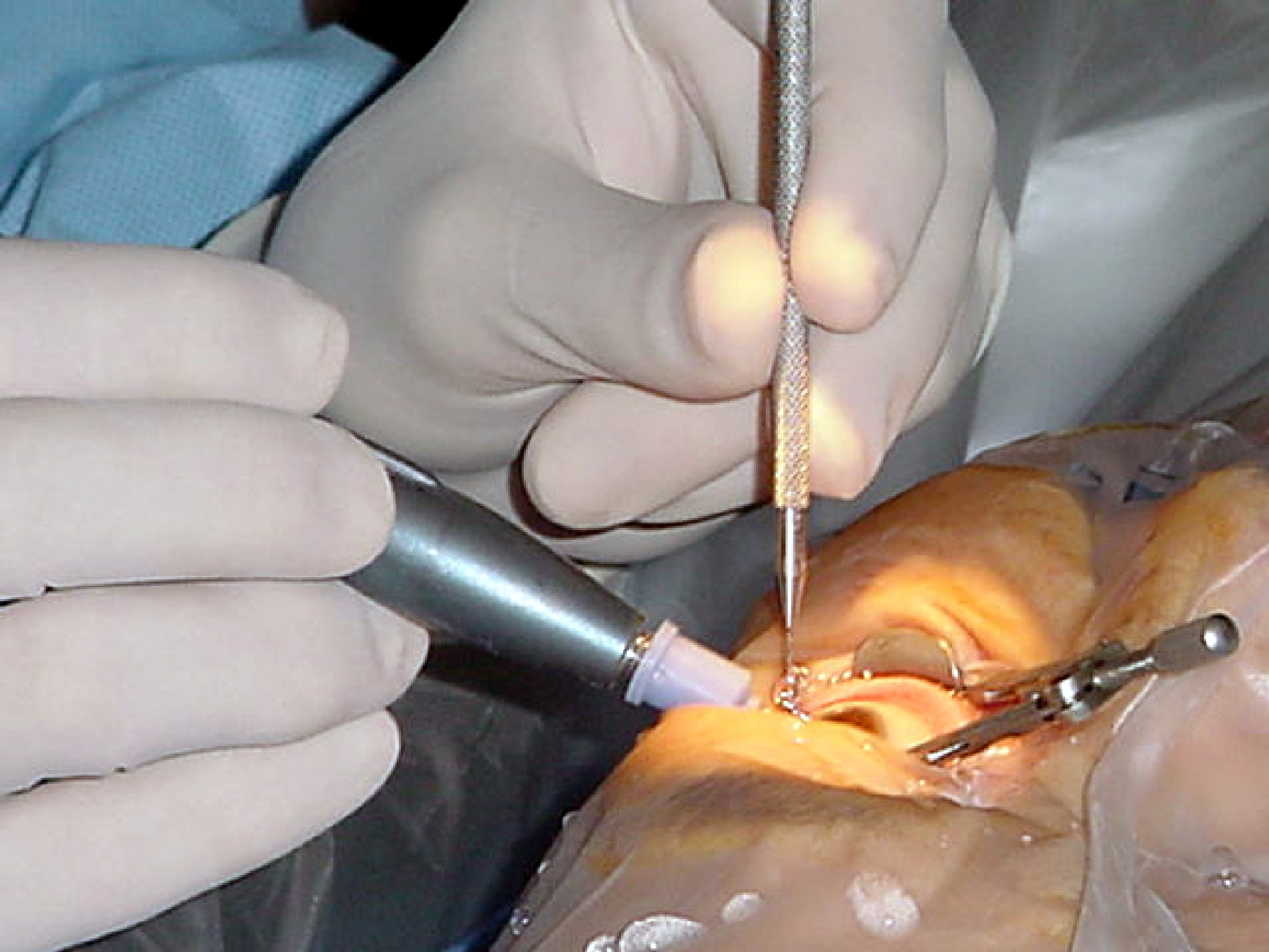
Cataract Surgery
Cataract surgery, also called lens replacement surgery, is the removal of the natural lens of the eye (also called "crystalline lens") that has developed an opacification, which is referred to as a cataract, and its replacement with an intraocular lens. Metabolic changes of the crystalline lens fibers over time lead to the development of the cataract, causing impairment or loss of vision. Some infants are born with congenital cataracts, and certain environmental factors may also lead to cataract formation. Early symptoms may include strong glare from lights and small light sources at night, and reduced acuity at low light levels. During cataract surgery, a patient's cloudy natural cataract lens is removed, either by emulsification in place or by cutting it out. An artificial intraocular lens (IOL) is implanted in its place. Cataract surgery is generally performed by an ophthalmologist in an ambulatory setting at a surgical center or hospital rather than an inpatient setting. Eit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
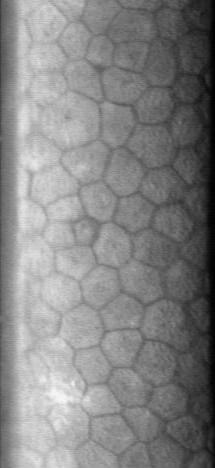
Corneal Endothelium
The corneal endothelium is a single layer of endothelial cells on the inner surface of the cornea. It faces the chamber formed between the cornea and the iris. The corneal endothelium are specialized, flattened, mitochondria-rich cells that line the posterior surface of the cornea and face the anterior chamber of the eye. The corneal endothelium governs fluid and solute transport across the posterior surface of the cornea and maintains the cornea in the slightly dehydrated state that is required for optical transparency. Embryology and anatomy The corneal endothelium is embryologically derived from the neural crest. The postnatal total endothelial cellularity of the cornea (approximately 300,000 cells per cornea) is achieved as early as the second trimester of gestation. Thereafter the endothelial cell density (but not the absolute number of cells) rapidly declines, as the fetal cornea grows in surface area, achieving a final adult density of approximately 2400 - 3200 cells ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
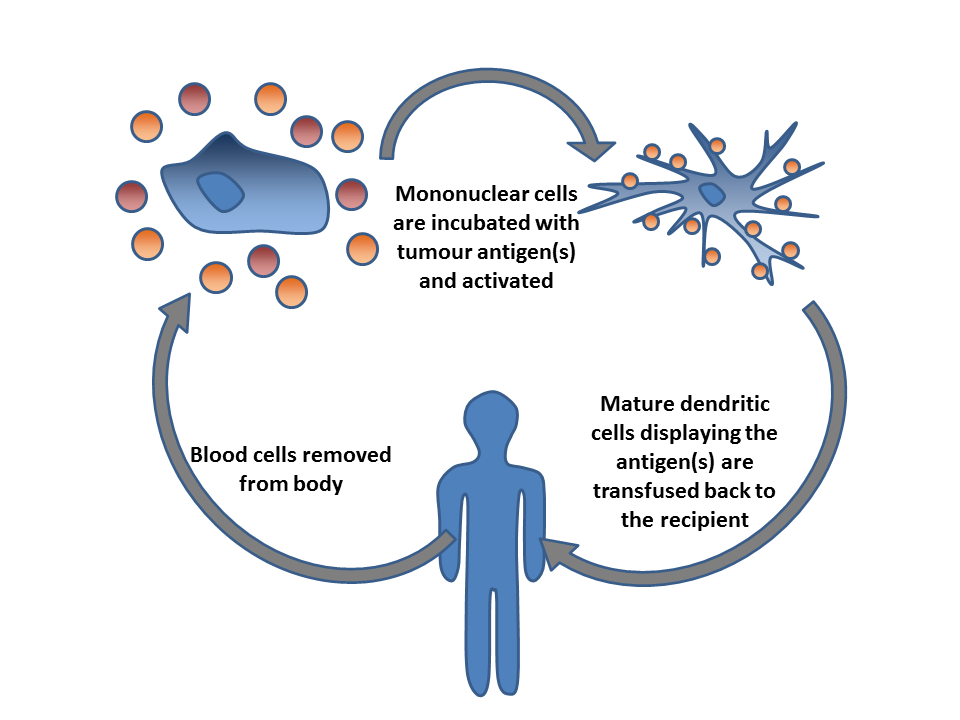
Immunooncology
Cancer immunotherapy (sometimes called immuno-oncology) is the stimulation of the immune system to treat cancer, improving on the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology. Cancer immunotherapy exploits the fact that cancer cells often have tumor antigens, molecules on their surface that can be detected by the antibody proteins of the immune system, binding to them. The tumor antigens are often proteins or other macromolecules (e.g., carbohydrates). Normal antibodies bind to external pathogens, but the modified immunotherapy antibodies bind to the tumor antigens marking and identifying the cancer cells for the immune system to inhibit or kill. Clinical success of cancer immunotherapy is highly variable between different forms of cancer; for instance, certain subtypes of gastric cancer react well to the approach whereas immunotherapy is not effective for other s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mitochondrial Myopathies
Mitochondrial myopathies are types of myopathies associated with mitochondrial disease. On biopsy, the muscle tissue of patients with these diseases usually demonstrate "ragged red" muscle fibers. These ragged-red fibers contain mild accumulations of glycogen and neutral lipids, and may show an increased reactivity for succinate dehydrogenase and a decreased reactivity for cytochrome c oxidase. Inheritance was believed to be maternal ( non-Mendelian extranuclear). It is now known that certain nuclear DNA deletions can also cause mitochondrial myopathy such as the OPA1 gene deletion. There are several subcategories of mitochondrial myopathies. Signs and symptoms Signs and symptoms include (for each of the following causes): * Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like syndrome (MELAS) ** Varying degrees of cognitive impairment and dementia ** Lactic acidosis ** Strokes ** Transient ischemic attacks ** Hearing loss ** Weight loss * Myoclonic epilepsy and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioenergetics
Bioenergetics is a field in biochemistry and cell biology that concerns energy flow through living systems. This is an active area of biological research that includes the study of the transformation of energy in living organisms and the study of thousands of different cellular processes such as cellular respiration and the many other metabolic and enzymatic processes that lead to production and utilization of energy in forms such as adenosine triphosphate (ATP) molecules. Nelson, David L., Cox, Michael M. ''Lehninger: Principles of Biochemistry.'' New York: W.H. Freeman and Company, 2013. Sixth ed., pg. 27. That is, the goal of bioenergetics is to describe how living organisms acquire and transform energy in order to perform biological work. Nelson, David L., Cox, Michael M. ''Lehninger: Principles of Biochemistry.'' New York: W.H. Freeman and Company, 2013. Sixth ed., pg. 24. The study of metabolic pathways is thus essential to bioenergetics. Overview Bioenergetics is the par ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |