|
Quantum Critical Point
A quantum critical point is a point in the phase diagram of a material where a continuous phase transition takes place at absolute zero. A quantum critical point is typically achieved by a continuous suppression of a nonzero temperature phase transition to zero temperature by the application of a pressure, field, or through doping. Conventional phase transitions occur at nonzero temperature when the growth of random thermal fluctuations leads to a change in the physical state of a system. Condensed matter physics research over the past few decades has revealed a new class of phase transitions called quantum phase transitions which take place at absolute zero. In the absence of the thermal fluctuations which trigger conventional phase transitions, quantum phase transitions are driven by the zero point quantum fluctuations associated with Heisenberg's uncertainty principle. Overview Within the class of phase transitions, there are two main categories: at a ''first-order phase t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Diagram
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium. Overview Common components of a phase diagram are ''lines of equilibrium'' or ''phase boundaries'', which refer to lines that mark conditions under which multiple phases can coexist at equilibrium. Phase transitions occur along lines of equilibrium. Metastable phases are not shown in phase diagrams as, despite their common occurrence, they are not equilibrium phases. Triple points are points on phase diagrams where lines of equilibrium intersect. Triple points mark conditions at which three different phases can coexist. For example, the water phase diagram has a triple point corresponding to the single temperature and pressure at which solid, liquid, and gaseous water can coexist in a stabl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antiferromagnetism
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins (on different sublattices) pointing in opposite directions. This is, like ferromagnetism and ferrimagnetism, a manifestation of ordered magnetism. The phenomenon of antiferromagnetism was first introduced by Lev Landau in 1933. Generally, antiferromagnetic order may exist at sufficiently low temperatures, but vanishes at and above the Néel temperature – named after Louis Néel, who had first identified this type of magnetic ordering. Above the Néel temperature, the material is typically paramagnetic. Measurement When no external field is applied, the antiferromagnetic structure corresponds to a vanishing total magnetization. In an external magnetic field, a kind of ferrimagnetic behavior may be displayed in the antiferromagnetic phase, with the absolute value of one of the sublattice magneti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
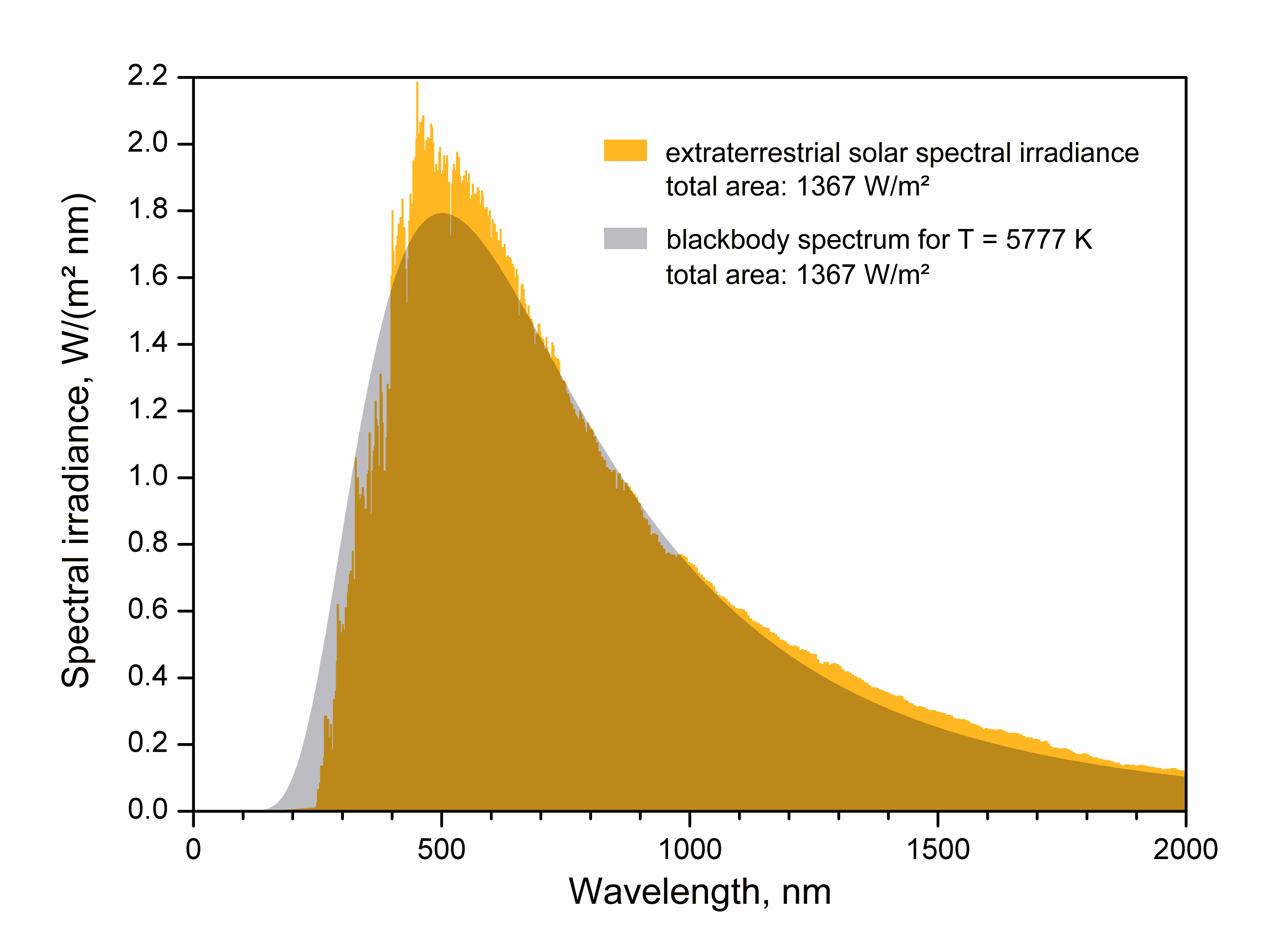
Effective Temperature
The effective temperature of a body such as a star or planet is the temperature of a black body that would emit the same total amount of electromagnetic radiation. Effective temperature is often used as an estimate of a body's surface temperature when the body's emissivity curve (as a function of wavelength) is not known. When the star's or planet's net emissivity in the relevant wavelength band is less than unity (less than that of a black body), the actual temperature of the body will be higher than the effective temperature. The net emissivity may be low due to surface or atmospheric properties, including greenhouse effect. Star The effective temperature of a star is the temperature of a black body with the same luminosity per ''surface area'' () as the star and is defined according to the Stefan–Boltzmann law . Notice that the total (bolometric) luminosity of a star is then , where is the stellar radius. The definition of the stellar radius is obviously not straightf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetostriction
Magnetostriction (cf. electrostriction) is a property of magnetic materials that causes them to change their shape or dimensions during the process of magnetization. The variation of materials' magnetization due to the applied magnetic field changes the magnetostrictive strain until reaching its saturation value, λ. The effect was first identified in 1842 by James Joule when observing a sample of iron. This effect causes energy loss due to frictional heating in susceptible ferromagnetic cores. The effect is also responsible for the low-pitched humming sound that can be heard coming from transformers, where oscillating AC currents produce a changing magnetic field. Explanation Internally, ferromagnetic materials have a structure that is divided into '' domains'', each of which is a region of uniform magnetization. When a magnetic field is applied, the boundaries between the domains shift and the domains rotate; both of these effects cause a change in the material's dimensions. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Effective Mass (solid-state Physics)
In solid state physics, a particle's effective mass (often denoted m^*) is the mass that it ''seems'' to have when responding to forces, or the mass that it seems to have when interacting with other identical particles in a thermal distribution. One of the results from the band theory of solids is that the movement of particles in a periodic potential, over long distances larger than the lattice spacing, can be very different from their motion in a vacuum. The effective mass is a quantity that is used to simplify band structures by modeling the behavior of a free particle with that mass. For some purposes and some materials, the effective mass can be considered to be a simple constant of a material. In general, however, the value of effective mass depends on the purpose for which it is used, and can vary depending on a number of factors. For electrons or electron holes in a solid, the effective mass is usually stated as a factor multiplying the rest mass of an electron, ''m'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metamagnetism
Metamagnetism is a sudden (often, dramatic) increase in the magnetization of a material with a small change in an externally applied magnetic field. The metamagnetic behavior may have quite different physical causes for different types of metamagnets. Some examples of physical mechanisms leading to metamagnetic behavior are: # Itinerant metamagnetism - Exchange splitting of the Fermi surface in a paramagnetic system of itinerant electrons causes an energetically favorable transition to bulk magnetization near the transition to a ferromagnet or other magnetically ordered state. # Antiferromagnetic transition - Field-induced spin flips in antiferromagnets cascade at a critical energy determined by the applied magnetic field. Depending on the material and experimental conditions, metamagnetism may be associated with a first-order phase transition In chemistry, thermodynamics, and other related fields, a phase transition (or phase change) is the physical process of transition bet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Critical Opalescence
Critical opalescence is a phenomenon which arises in the region of a continuous, or second-order, phase transition. Originally reported by Charles Cagniard de la Tour in 1823 in mixtures of alcohol and water, its importance was recognised by Thomas Andrews in 1869 following his experiments on the liquid-gas transition in carbon dioxide; many other examples have been discovered since. The phenomenon is most commonly demonstrated in binary fluid mixtures, such as methanol and cyclohexane. As the critical point is approached, the sizes of the gas and liquid region begin to fluctuate over increasingly large length scales (the correlation length of the liquid diverges). As the density fluctuations become of a size comparable to the wavelength of light, the light is scattered and causes the normally transparent liquid to appear cloudy. Tellingly, the opalescence does not diminish as one gets closer to the critical point, where the largest fluctuations can reach even centimetre proport ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superconductivity
Superconductivity is a set of physical properties observed in certain materials where electrical resistance vanishes and magnetic flux fields are expelled from the material. Any material exhibiting these properties is a superconductor. Unlike an ordinary metallic conductor, whose resistance decreases gradually as its temperature is lowered even down to near absolute zero, a superconductor has a characteristic critical temperature below which the resistance drops abruptly to zero. An electric current through a loop of superconducting wire can persist indefinitely with no power source. The superconductivity phenomenon was discovered in 1911 by Dutch physicist Heike Kamerlingh Onnes. Like ferromagnetism and atomic spectral lines, superconductivity is a phenomenon which can only be explained by quantum mechanics. It is characterized by the Meissner effect, the complete ejection of magnetic field lines from the interior of the superconductor during its transitions into the sup ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermi Liquid Theory
Fermi liquid theory (also known as Landau's Fermi-liquid theory) is a theoretical model of interacting fermions that describes the normal state of most metals at sufficiently low temperatures. The interactions among the particles of the many-body system do not need to be small. The phenomenological theory of Fermi liquids was introduced by the Soviet physicist Lev Davidovich Landau in 1956, and later developed by Alexei Abrikosov and Isaak Khalatnikov using diagrammatic perturbation theory. The theory explains why some of the properties of an interacting fermion system are very similar to those of the ideal Fermi gas (i.e. non-interacting fermions), and why other properties differ. Important examples of where Fermi liquid theory has been successfully applied are most notably electrons in most metals and liquid helium-3. Liquid helium-3 is a Fermi liquid at low temperatures (but not low enough to be in its superfluid phase). Helium-3 is an isotope of helium, with 2 protons, 1 neu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermi Liquid
Fermi liquid theory (also known as Landau's Fermi-liquid theory) is a theoretical model of interacting fermions that describes the normal state of most metals at sufficiently low temperatures. The interactions among the particles of the many-body system do not need to be small. The phenomenological theory of Fermi liquids was introduced by the Soviet physicist Lev Davidovich Landau in 1956, and later developed by Alexei Abrikosov and Isaak Khalatnikov using diagrammatic perturbation theory. The theory explains why some of the properties of an interacting fermion system are very similar to those of the ideal Fermi gas (i.e. non-interacting fermions), and why other properties differ. Important examples of where Fermi liquid theory has been successfully applied are most notably electrons in most metals and liquid helium-3. Liquid helium-3 is a Fermi liquid at low temperatures (but not low enough to be in its superfluid phase). Helium-3 is an isotope of helium, with 2 protons, 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferromagnetism
Ferromagnetism is a property of certain materials (such as iron) which results in a large observed magnetic permeability, and in many cases a large magnetic coercivity allowing the material to form a permanent magnet. Ferromagnetic materials are the familiar metals noticeably attracted to a magnet, a consequence of their large magnetic permeability. Magnetic permeability describes the induced magnetization of a material due to the presence of an ''external'' magnetic field, and it is this temporarily induced magnetization inside a steel plate, for instance, which accounts for its attraction to the permanent magnet. Whether or not that steel plate acquires a permanent magnetization itself, depends not only on the strength of the applied field, but on the so-called coercivity of that material, which varies greatly among ferromagnetic materials. In physics, several different types of material magnetism are distinguished. Ferromagnetism (along with the similar effect ferrimagnetis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Transition
In chemistry, thermodynamics, and other related fields, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states of matter: solid, liquid, and gas, and in rare cases, plasma. A phase of a thermodynamic system and the states of matter have uniform physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point. Types of phase transition At the phase transition point for a substance, for instance the boiling point, the two phases involved - liquid and vapor, have identic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |