|
Pseudoisoeugenol
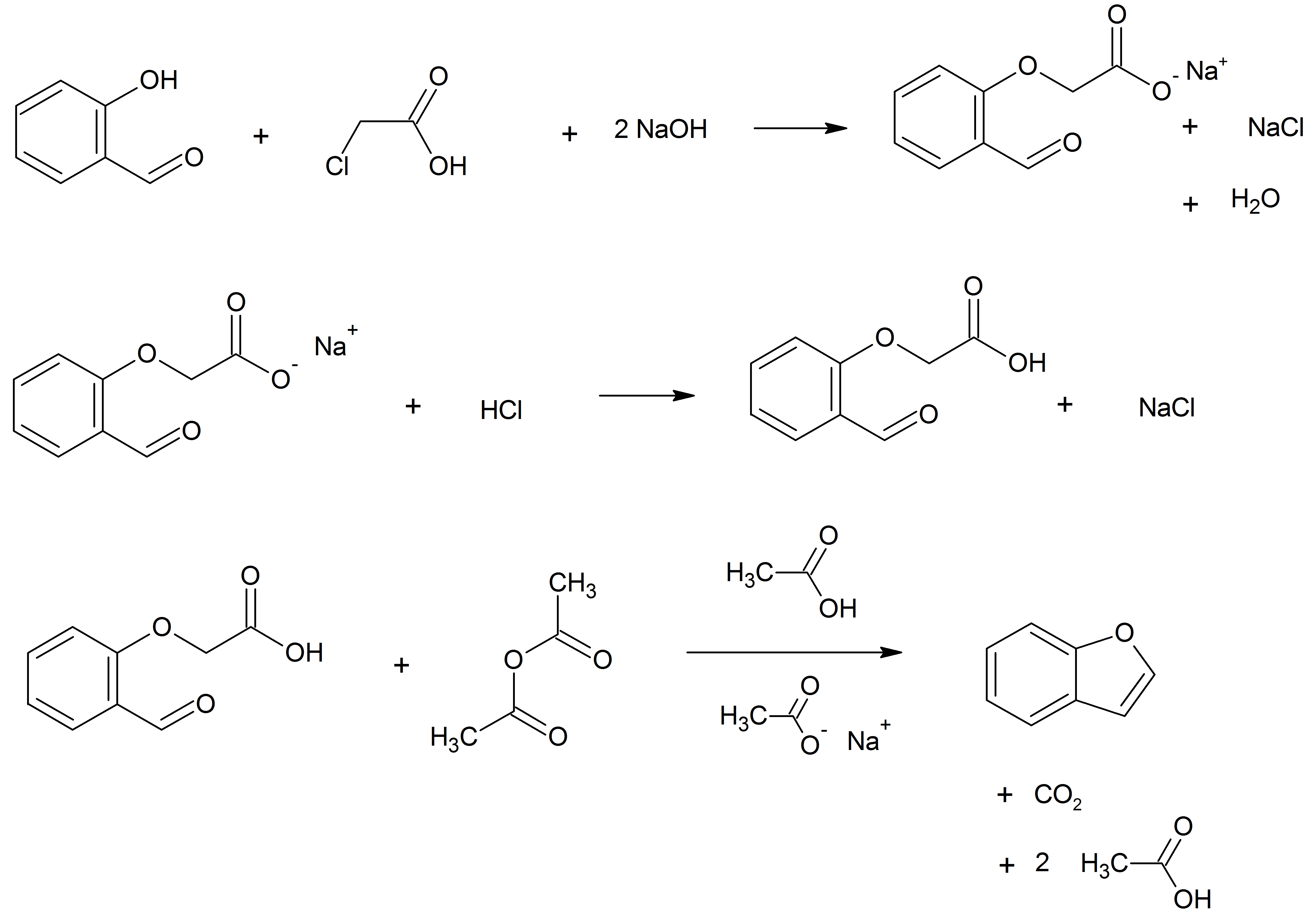
Pseudoisoeugenol is a naturally occurring phenylpropene and an isomer of eugenol. Natural occurrence and derivatives Pseudoisoeugenol naturally occurs in the essential oils of roots from plants within the genus ''Pimpinella''. In addition to its standard form, the compound also occurs in a variety of structural derivatives. Common derivatives include the compound with its side chain bearing an epoxide functional group and the aromatic ring being associated with one of many possible esters in the 2nd position. Common esters include angelic acid, 2-methylbutanoic acid, tiglic acid, and 2-methylpropionic acid esters. Hydrolysis of these esters, either ''in-vivo'' or by using strong acids, forms 2-methyl-5-methoxybenzofuran. Biosynthesis Biosynthesis of the compound is hypothesized to proceed via a NIH shift of anethole. See also *Isoeugenol Isoeugenol is a phenylpropene, a propenyl-substituted guaiacol. A phenylpropanoid, it occurs in the essential oils of plants such as ylang- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eugenol
Eugenol is an allyl chain-substituted guaiacol, a member of the allylbenzene class of chemical compounds. It is a colorless to pale yellow, aromatic oily liquid extracted from certain essential oils especially from clove, nutmeg, cinnamon, basil and bay leaf. It is present in concentrations of 80–90% in clove bud oil and at 82–88% in clove leaf oil. Eugenol has a pleasant, spicy, clove-like scent. The name is derived from ''Eugenia caryophyllata'', the former Linnean nomenclature term for cloves. The currently accepted name is ''Syzygium aromaticum''. Biosynthesis The biosynthesis of eugenol begins with the amino acid tyrosine. L-tyrosine is converted to ''p''-coumaric acid by the enzyme tyrosine ammonia lyase (TAL). From here, ''p''-coumaric acid is converted to caffeic acid by ''p''-coumarate 3-hydroxylase using oxygen and NADPH. ''S''-Adenosyl methionine (SAM) is then used to methylate caffeic acid, forming ferulic acid, which is in turn converted to feruloyl- CoA by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoeugenol
Isoeugenol is a phenylpropene, a propenyl-substituted guaiacol. A phenylpropanoid, it occurs in the essential oils of plants such as ylang-ylang (''Cananga odorata''), and is a component of wood smoke and liquid smoke. It can be synthesized from eugenol and has been used in the manufacture of vanillin. It may occur as either the cis ''(Z)'' or trans ''(E)'' isomer. Trans ''(E)'' isoeugenol is crystalline while cis ''(Z)'' isoeugenol is a liquid. Isoeugenol is one of several phenolic compounds responsible for the mold-inhibiting effect of smoke on meats and cheeses. Allergy Some individuals experience a hives-like reaction to long-term exposure to Isoeugenol, which is named as ''Fragrance'' in the ingredients of consumer products such as soaps, shampoos and detergents, bath tissue, and topical cosmetic applications. Sensitivity to isoeugenol (Fragrance) may be identified with a clinical patch test. See also * Pseudoisoeugenol * Methylisoeugenol * Desmethylisoeugenol Hydroxychavi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anethole
Anethole (also known as anise camphor) is an organic compound that is widely used as a flavoring substance. It is a derivative of phenylpropene, a type of aromatic compound that occurs widely in nature, in essential oils. It is in the class of phenylpropanoid organic compounds. It contributes a large component of the odor and flavor of anise and fennel (both in the botanical family Apiaceae), anise myrtle ( Myrtaceae), liquorice ( Fabaceae), magnolia blossoms, and star anise (Schisandraceae). Closely related to anethole is its isomer estragole, abundant in tarragon (Asteraceae) and basil (Lamiaceae), that has a flavor reminiscent of anise. It is a colorless, fragrant, mildly volatile liquid. Anethole is only slightly soluble in water but exhibits high solubility in ethanol. This trait causes certain anise-flavored liqueurs to become opaque when diluted with water; the ouzo effect. Structure and production Anethole is an aromatic, unsaturated ether related to lignols. It e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylpropene
Phenylpropene is the organic compound with the formula C6H5CH2CH=CH2. It is a colorless liquid. The compound consists of a phenyl group attached to allyl. Phenylpropene isomerizes to trans-propenylbenzene. In plant biochemistry, the phenylpropene skeleton is the parent (simplest representation) of the phenylpropanoids. Prominent derivatives include eugenol, safrole Safrole is an organic compound with the formula CH2O2C6H3CH2CH=CH2. It is a colorless oily liquid, although impure samples can appear yellow. A member of the phenylpropanoid family of natural products, it is found in sassafras plants, among oth ..., and many others. References External links * {{Phenylpropene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-methylpropionic Acid
Isobutyric acid, also known as 2-methylpropanoic acid or isobutanoic acid, is a carboxylic acid with structural formula ( CH3)2CH COOH. It is an isomer of ''n''- butyric acid. It is classified as a short-chain fatty acid. Deprotonation or esterification gives derivatives called isobutyrates. Isobutyric acid is a colorless liquid with a somewhat unpleasant odor. It is soluble in water and organic solvents. It is found naturally in carobs (''Ceratonia siliqua''), in vanilla, and in the root of ''Arnica dulcis'', and as an ethyl ester in croton oil. Production Isobutyric acid is manufactured by the oxidation of isobutyraldehyde, which is a byproduct of the hydroformylation of propylene. It can also be prepared by the high pressure hydrocarboxylation ( Koch reaction) from propylene: :CH3CH=CH2 + CO + H2O → (CH3)2CHCO2H Isobutyric acid can also be manufactured commercially using engineered bacteria with a sugar feedstock. Laboratory methods Many routes are known incl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylpropenes
Phenylpropene is the organic compound with the formula C6H5CH2CH=CH2. It is a colorless liquid. The compound consists of a phenyl group attached to allyl. Phenylpropene isomerizes to trans-propenylbenzene. In plant biochemistry, the phenylpropene skeleton is the parent (simplest representation) of the phenylpropanoids. Prominent derivatives include eugenol, safrole Safrole is an organic compound with the formula CH2O2C6H3CH2CH=CH2. It is a colorless oily liquid, although impure samples can appear yellow. A member of the phenylpropanoid family of natural products, it is found in sassafras plants, among oth ..., and many others. References External links * {{Phenylpropene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NIH Shift
An NIH shift is a chemical rearrangement where a hydrogen atom on an aromatic ring undergoes an intramolecular migration primarily during a hydroxylation reaction. This process is also known as a 1,2-hydride shift. These shifts are often studied and observed by isotopic labeling. An example of an NIH shift is shown below: In this example, a hydrogen atom has been isotopically labeled using deuterium (shown in red). As the hydroxylase adds a hydroxyl (the −OH group), the labeled site shifts one position around the aromatic ring relative to the stationary methyl group (−CH3). Several hydroxylase enzymes are believed to incorporate an NIH shift in their mechanism, including 4-hydroxyphenylpyruvate dioxygenase and the tetrahydrobiopterin dependent hydroxylases. The name ''NIH shift'' arises from the US National Institutes of Health The National Institutes of Health, commonly referred to as NIH (with each letter pronounced individually), is the primary agency of the Unite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzofuran
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. *Perkin rearrangement, where a coumarin is reacted with a hydroxide: : * Diels–Alder reaction of nitro vinyl furans with various dienophiles: : Diels–Alder reaction yielding a substituted benzofuran, 450px * Cycloisomerization of alkyne ortho-substituted phenols: : Benzofurans via Cycloisomerization, 400px Related com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strong Acid
Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H+, and an anion, A-. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions. :HA -> H+ + A- Examples of strong acids are hydrochloric acid (HCl), perchloric acid (HClO4), nitric acid (HNO3) and sulfuric acid (H2SO4). A weak acid is only partially dissociated, with both the undissociated acid and its dissociation products being present, in solution, in equilibrium with each other. :HA H+ + A- Acetic acid (CH3COOH) is an example of a weak acid. The strength of a weak acid is quantified by its acid dissociation constant, K_\ce value. The strength of a weak organic acid may depend on substituent effects. The strength of an inorganic acid is dependent on the oxidation state for the atom to which the proton may be attached. Acid strength is solvent-dependent. For example, hydrogen chloride is a strong acid in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
In-vivo
Studies that are ''in vivo'' (Latin for "within the living"; often not italicized in English) are those in which the effects of various biological entities are tested on whole, living organisms or cells, usually animals, including humans, and plants, as opposed to a tissue extract or dead organism. This is not to be confused with experiments done ''in vitro'' ("within the glass"), i.e., in a laboratory environment using test tubes, Petri dishes, etc. Examples of investigations ''in vivo'' include: the pathogenesis of disease by comparing the effects of bacterial infection with the effects of purified bacterial toxins; the development of non-antibiotics, antiviral drugs, and new drugs generally; and new surgical procedures. Consequently, animal testing and clinical trials are major elements of ''in vivo'' research. ''In vivo'' testing is often employed over ''in vitro'' because it is better suited for observing the overall effects of an experiment on a living subject. In drug d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature Etymology Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |