|
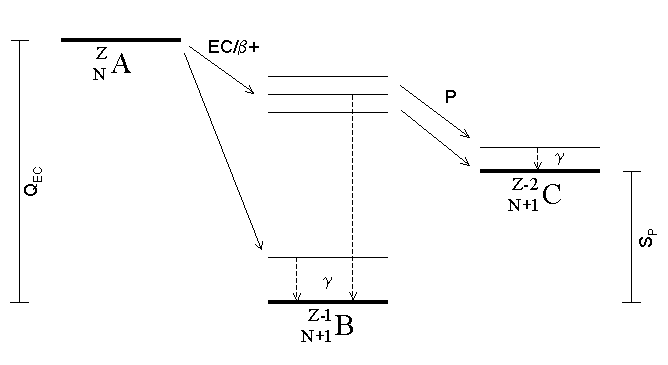
Proton Emission
Proton emission (also known as proton radioactivity) is a rare type of radioactive decay in which a proton is ejected from a nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay, in which case the process is known as beta-delayed proton emission, or can occur from the ground state (or a low-lying isomer) of very proton-rich nuclei, in which case the process is very similar to alpha decay. For a proton to escape a nucleus, the proton separation energy must be negative—the proton is therefore unbound, and tunnels out of the nucleus in a finite time. Proton emission is not seen in naturally occurring isotopes; proton emitters can be produced via nuclear reactions, usually using linear particle accelerators. Although prompt (i.e. not beta-delayed) proton emission was observed from an isomer in cobalt-53 as early as 1969, no other proton-emitting states were found until 1981, when the proton radioactive ground states of lutetium-15 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron-45
Naturally occurring iron (26Fe) consists of four stable isotopes: 5.845% of 54Fe (possibly radioactive with a half-life over years), 91.754% of 56Fe, 2.119% of 57Fe and 0.286% of 58Fe. There are 24 known radioactive isotopes, the most stable of which are 60Fe (half-life 2.6 million years) and 55Fe (half-life 2.7 years). Much of the past work on measuring the isotopic composition of Fe has centered on determining 60Fe variations due to processes accompanying nucleosynthesis (i.e., meteorite studies) and ore formation. In the last decade however, advances in mass spectrometry technology have allowed the detection and quantification of minute, naturally occurring variations in the ratios of the stable isotopes of iron. Much of this work has been driven by the Earth and planetary science communities, although applications to biological and industrial systems are beginning to emerge. List of isotopes , - , rowspan=2, 45Fe , rowspan=2 style="text-align:right" , 26 , rowspan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Physics
Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions, in addition to the study of other forms of nuclear matter. Nuclear physics should not be confused with atomic physics, which studies the atom as a whole, including its electrons. Discoveries in nuclear physics have led to applications in many fields. This includes nuclear power, nuclear weapons, nuclear medicine and magnetic resonance imaging, industrial and agricultural isotopes, ion implantation in materials engineering, and radiocarbon dating in geology and archaeology. Such applications are studied in the field of nuclear engineering. Particle physics evolved out of nuclear physics and the two fields are typically taught in close association. Nuclear astrophysics, the application of nuclear physics to astrophysics, is crucial in explaining the inner workings of stars and the origin of the chemical elements. History The history of nuclear physics as a discipl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photodisintegration
Photodisintegration (also called phototransmutation, or a photonuclear reaction) is a nuclear process in which an atomic nucleus absorbs a high-energy gamma ray, enters an excited state, and immediately decays by emitting a subatomic particle. The incoming gamma ray effectively knocks one or more neutrons, protons, or an alpha particle out of the nucleus. The reactions are called (γ,n), (γ,p), and (γ,α). Photodisintegration is endothermic (energy absorbing) for atomic nuclei lighter than iron and sometimes exothermic (energy releasing) for atomic nuclei heavier than iron. Photodisintegration is responsible for the nucleosynthesis of at least some heavy, proton-rich elements via the p-process in supernovae. This causes the iron to further fuse into the heavier elements. Photodisintegration of deuterium A photon carrying 2.22 MeV or more energy can photodisintegrate an atom of deuterium: : James Chadwick and Maurice Goldhaber used this reaction to measure the proton-neutron mass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Emission
Neutron emission is a mode of radioactive decay in which one or more neutrons are ejected from a nucleus. It occurs in the most neutron-rich/proton-deficient nuclides, and also from excited states of other nuclides as in photoneutron emission and beta-delayed neutron emission. As only a neutron is lost by this process the number of protons remains unchanged, and an atom does not become an atom of a different element, but a different isotope of the same element. Neutrons are also produced in the spontaneous and induced fission of certain heavy nuclides. Spontaneous neutron emission As a consequence of the Pauli exclusion principle, nuclei with an excess of protons or neutrons have a higher average energy per nucleon. Nuclei with a sufficient excess of neutrons have a greater energy than the combination of a free neutron and a nucleus with one less neutron, and therefore can decay by neutron emission. Nuclei which can decay by this process are described as lying beyond the neutron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free Neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Their properties and interactions are described by nuclear physics. Protons and neutrons are not elementary particles; each is composed of three quarks. The chemical properties of an atom are mostly determined by the configuration of electrons that orbit the atom's heavy nucleus. The electron configuration is determined by the charge of the nucleus, which is determined by the number of protons, or atomic number. The number of neutrons is the neutron number. Neutrons do not affect the electron configuration, but the sum of atomic and neutron numbers is the mass of the nucleus. Atoms of a chemical element that di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diproton
Although there are nine known isotopes of helium (2He) (standard atomic weight: ), only helium-3 () and helium-4 () are stable. All radioisotopes are short-lived, the longest-lived being with a half-life of . The least stable is , with a half-life of (), although it is possible that may have an even shorter half-life. In the Earth's atmosphere, the ratio of to is . However, the isotopic abundance of helium varies greatly depending on its origin. In the Local Interstellar Cloud, the proportion of to is , which is times higher than that of atmospheric helium. Rocks from the Earth's crust have isotope ratios varying by as much as a factor of ten; this is used in geology to investigate the origin of rocks and the composition of the Earth's mantle. The different formation processes of the two stable isotopes of helium produce the differing isotope abundances. Equal mixtures of liquid and below separate into two immiscible phases due to differences in quantum statistics: at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Drip Line
The nuclear drip line is the boundary beyond which atomic nuclei decay by the emission of a proton or neutron. An arbitrary combination of protons and neutrons does not necessarily yield a stable nucleus. One can think of moving up and/or to the right across the table of nuclides by adding one type of nucleon to a given nucleus. However, adding nucleons one at a time to a given nucleus will eventually lead to a newly formed nucleus that immediately decays by emitting a proton (or neutron). Colloquially speaking, the nucleon has ''leaked'' or ''dripped'' out of the nucleus, hence giving rise to the term ''drip line''. Drip lines are defined for protons and neutrons at the extreme of the proton-to-neutron ratio; at p:n ratios at or beyond the drip lines, no bound nuclei can exist. While the location of the proton drip line is well known for many elements, the location of the neutron drip line is only known for elements up to neon. General description Nuclear stability is limit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc-54
Naturally occurring zinc (30Zn) is composed of the 5 stable isotopes 64Zn, 66Zn, 67Zn, 68Zn, and 70Zn with 64Zn being the most abundant (48.6% natural abundance). Twenty-five radioisotopes have been characterised with the most abundant and stable being 65Zn with a half-life of 244.26 days, and 72Zn with a half-life of 46.5 hours. All of the remaining radioactive isotopes have half-lives that are less than 14 hours and the majority of these have half-lives that are less than 1 second. This element also has 10 meta states. Zinc has been proposed as a " salting" material for nuclear weapons. A jacket of isotopically enriched 64Zn, irradiated by the intense high-energy neutron flux from an exploding thermonuclear weapon, would transmute into the radioactive isotope 65Zn with a half-life of 244 days and produce approximately 1.115 MeV of gamma radiation, significantly increasing the radioactivity of the weapon's fallout for several years. Such a weapon is not known to have ever ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caen
Caen (, ; nrf, Kaem) is a commune in northwestern France. It is the prefecture of the department of Calvados. The city proper has 105,512 inhabitants (), while its functional urban area has 470,000,Comparateur de territoire INSEE, retrieved 20 June 2022. making Caen the second largest urban area in and the 19th largest in France. It is also the third largest commune in all of Normandy after and Rouen. It is located inland ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grand Accélérateur National D'Ions Lourds
The Grand Accélérateur National d’Ions Lourds (GANIL), or Large Heavy Ion National Accelerator, is a French national nuclear physics research center in Caen. The facility has been in operation since 1983, and consists primarily of two serialised synchrocyclotrons. See also Projects: * Fazia Similar facilities: * GSI * Riken, Japan * NSCL, USA * Dubna Dubna ( rus, Дубна́, p=dʊbˈna) is a town in Moscow Oblast, Russia. It has a status of ''naukograd'' (i.e. town of science), being home to the Joint Institute for Nuclear Research, an international nuclear physics research center and one o ..., Russia * CERN * TRIUMF External links GANILScholarpedia article {{DEFAULTSORT:Grand Accelerateur National d'Ions Lourds Laboratories in France Nuclear research institutes Research institutes in France French National Centre for Scientific Research Institutes associated with CERN ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |