|
Potassium Nonahydridorhenate
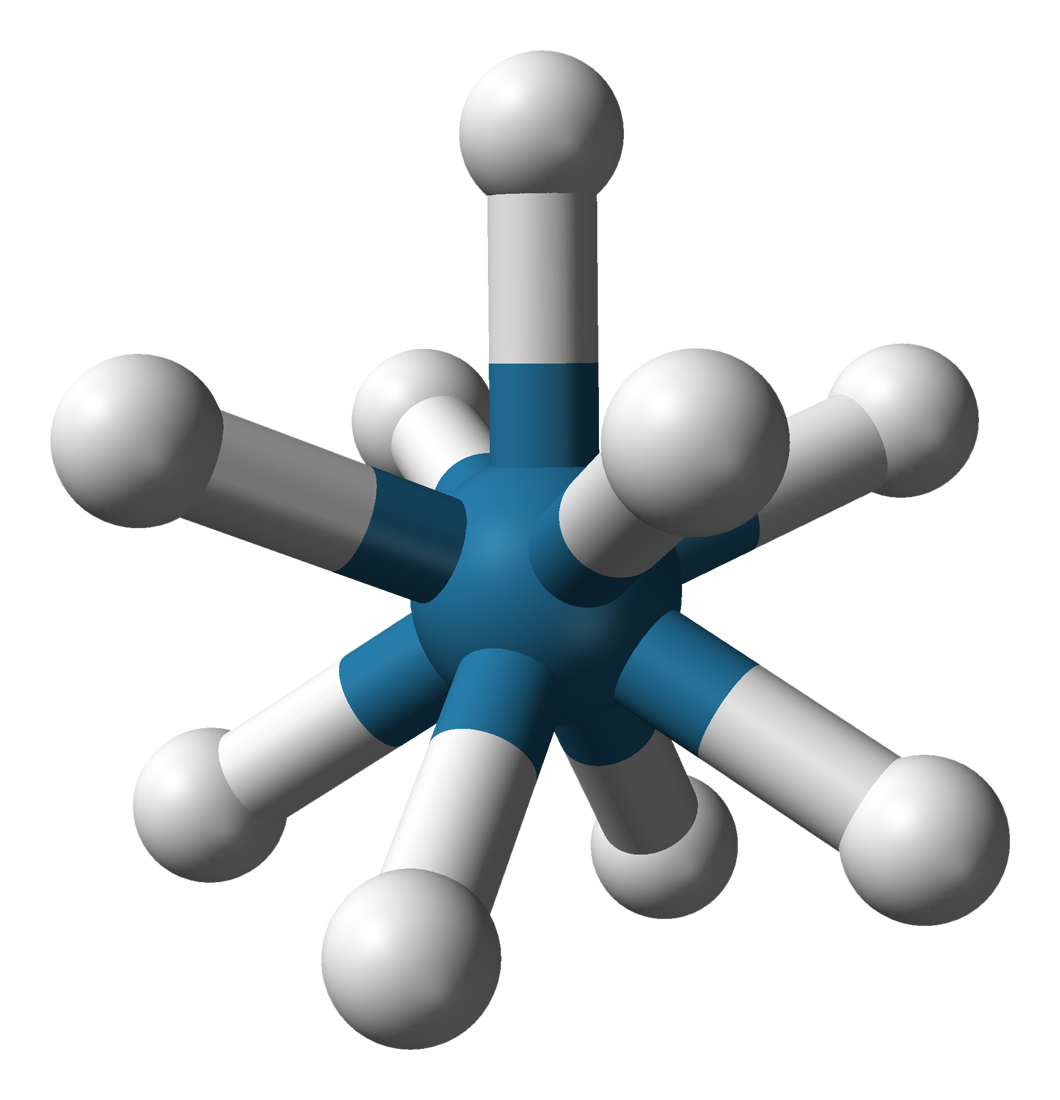
Potassium nonahydridorhenate(VII) is an inorganic compound having the formula K2ReH9. This colourless salt is soluble in water but only poorly soluble in most alcohols. The anion is a rare example of a coordination complex bearing only hydride ligands. History The study of rhenium hydrides can be traced to the 1950s and included reports of the "rhenide" anion, supposedly Re−. These reports led to a series of investigations by A. P. Ginsberg and coworkers on the products from the reduction of perrhenate. The ''rhenide'' anion, Re−, was based on the product of the reduction of perrhenate salts, such as the reduction of potassium perrhenate () by potassium metal. "Potassium rhenide" was shown to exist as a tetrahydrated complex, with the postulated chemical formula . This compound exhibits strongly reducing properties, and slowly yields hydrogen gas when dissolved in water. The lithium and thallous salts were also reported. Later research, however, indicates that the "rhen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting point of modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zeitschrift Für Anorganische Und Allgemeine Chemie
The ''Zeitschrift für anorganische und allgemeine Chemie'' (''Journal of Inorganic and General Chemistry'') is a semimonthly peer-reviewed scientific journal covering inorganic chemistry, published by Wiley-VCH. The editors-in-chief are Thomas F. Fässler, Christian Limberg, Guodong Qian, and David Scheschkewitz. Originally the journal was published in German, but nowadays it is completely in English. Abstracting and indexing The journal is abstracted and indexed in the following databases: According to the ''Journal Citation Reports'', the journal has a 2021 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as i ... of 1.414, ranking it 40th out of 46 journals in the category "Chemistry, Inorganic & Nuclear". References External links * Chemistry journals Wiley-VCH aca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Hydrides
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed. Almost all of the elements form binary compounds with hydrogen, the exceptions being He, Ne, Ar, Kr, Pm, Os, Ir, Rn, Fr, and Ra. Exotic molecules such as positronium hydride have also been made. Bonds Bonds between hydrogen and the other elements range from highly to somewhat covalent. Some hydrides, e.g. boron hydrides, do not conform to classical electron-counting rules and the bonding is described in terms of multi-centered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Turnstile
A turnstile (also called a turnpike, gateline, baffle gate, automated gate, turn gate in some regions) is a form of gate which allows one person to pass at a time. A turnstile can be configured to enforce one-way human traffic. In addition, a turnstile can restrict passage only to people who insert a coin, ticket, pass, or other method of payment. Modern turnstiles incorporate biometrics, including retina scanning, fingerprints, and other individual human characteristics which can be scanned. Thus a turnstile can be used in the case of paid access (sometimes called a faregate or ticket barrier when used for this purpose), for example to access public transport, a pay toilet, or to restrict access to authorized people, for example in the lobby of an office building. History Turnstiles were originally used, like other forms of stile, to allow human beings to pass while keeping sheep or other livestock penned in. The use of turnstiles in most modern applications has been credit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dance (Matisse)
''Dance'' (''La Danse'') is a painting made by Henri Matisse in 1910, at the request of Russian businessman and art collector Sergei Shchukin, who bequeathed the large decorative panel to the Hermitage Museum in Saint Petersburg, Russia. The composition of dancing figures is commonly recognized as "a key point of (Matisse's) career and in the development of modern painting". A preliminary version of the work, sketched by Matisse in 1909 as a study for the work, resides at MoMA in New York City, where it has been labeled ''Dance (I)''. ''La Danse'' was first exhibited at the Salon d'Automne of 1910 (1 October – 8 November), Grand Palais des Champs-Élysées, Paris. ''Dance (I)'' In March 1909, Matisse painted a preliminary version of this work, known as ''Dance (I)''. It was a compositional study and uses paler colors and less detail. The painting was highly regarded by the artist who once called it "the overpowering climax of luminosity"; it is also featured in the backgr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Circle Dance
Circle dance, or chain dance, is a style of social dance done in a circle, semicircle or a curved line to musical accompaniment, such as rhythm instruments and singing, and is a type of dance where anyone can join in without the need of partners. Unlike line dancing, circle dancers are in physical contact with each other; the connection is made by hand-to-hand, finger-to-finger or hands-on-shoulders, where they follow the leader around the dance floor. Ranging from gentle to energetic, the dance can be an uplifting group experience or part of a meditation. Being probably the oldest known dance formation, circle dancing is an ancient tradition common to many cultures for marking special occasions, rituals, strengthening community and encouraging togetherness. Circle dances are choreographed to many different styles of music and rhythms. Modern circle dance mixes traditional folk dances, mainly from European or Near Eastern sources, with recently choreographed ones to a va ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition State
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked with the double dagger ‡ symbol. As an example, the transition state shown below occurs during the SN2 reaction of bromoethane with a hydroxide anion: The activated complex of a reaction can refer to either the transition state or to other states along the reaction coordinate between reactants and products, especially those close to the transition state.Peter Atkins and Julio de Paula, ''Physical Chemistry'' (8th ed., W.H. Freeman 2006), p.809 According to the transition state theory, once the reactants have passed through the transition state configuration, they always continue to form products. History of concept The concept of a transition state has been important in many theories of the rates at which chemical reactions occ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capped Square Antiprismatic Molecular Geometry
In chemistry, the capped square antiprismatic molecular geometry describes the shape of compounds where nine atoms, groups of atoms, or ligands are arranged around a central atom, defining the vertices of a gyroelongated square pyramid. The gyroelongated square pyramid is a square pyramid with a square antiprism connected to the square base. In this respect, it can be seen as a "capped" square antiprism (a square antiprism with a pyramid erected on one of the square faces). It is very similar to the tricapped trigonal prismatic molecular geometry, and there is some dispute over the specific geometry exhibited by certain molecules. Examples * is sometimes described as having a capped square antiprismatic geometry, although its geometry is most often described as tricapped trigonal prismatic. *, a lanthanum Lanthanum is a chemical element with the symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraethylammonium
Tetraethylammonium (TEA), () or (Et4N+) is a quaternary ammonium cation consisting of four ethyl groups attached to a central nitrogen atom, and is positively charged. It is a counterion used in the research laboratory to prepare lipophilic salts of inorganic anions. It is used similarly to tetrabutylammonium, the difference being that its salts are less lipophilic and more easily crystallized. Preparation The chloride salt is prepared by the reaction of triethylamine and an ethyl halide: :Et3N + EtX → Et4N+X− This method works well for the preparation of tetraethylammonium iodide (where X = I). Most tetraethylammonium salts are prepared by salt metathesis reactions. For example, the synthesis of tetraethylammonium perchlorate, a salt that has been useful as a supporting electrolyte for polarographic studies in non-aqueous solvents, is carried out by mixing the water-soluble salts tetraethylammonium bromide and sodium perchlorate in water, from which the water-insoluble tet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Perrhenate
Sodium perrhenate (also known as sodium rhenate(VII)) is the inorganic compound with the formula NaReO4. It is a white salt that is soluble in water. It is a common precursor to other rhenium compounds. Its structure resembles that of sodium perchlorate and sodium permanganate. Preparation It can be prepared by treatment of rhenium heptoxide with base or by ion exchange Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, ... from the potassium salt. Sodium perrhenate can be prepared from rhenium metal with hydrogen peroxide in the presence of base. :2 Re + 7 H2O2 + 2 NaOH -> 2 NaReO4 + 8 H2O Reactions It reacts with sodium in ethanol to give nonahydridorhenate. Sodium perrhenate has been used as a precursor of rhenium nitrides (such as Re3N, Re2N, Re3N2, ReN2, ReN3, ReN4), which c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Technetium
Technetium is a chemical element with the symbol Tc and atomic number 43. It is the lightest element whose isotopes are all radioactive. All available technetium is produced as a synthetic element. Naturally occurring technetium is a spontaneous fission product in uranium ore and thorium ore, the most common source, or the product of neutron capture in molybdenum ores. This silvery gray, crystalline transition metal lies between manganese and rhenium in group 7 of the periodic table, and its chemical properties are intermediate between those of both adjacent elements. The most common naturally occurring isotope is 99Tc, in traces only. Many of technetium's properties had been predicted by Dmitri Mendeleev before it was discovered. Mendeleev noted a gap in his periodic table and gave the undiscovered element the provisional name '' ekamanganese'' (''Em''). In 1937, technetium (specifically the technetium-97 isotope) became the first predominantly artificial element to be produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamagnetic
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted by a magnetic field. Diamagnetism is a quantum mechanical effect that occurs in all materials; when it is the only contribution to the magnetism, the material is called diamagnetic. In paramagnetic and ferromagnetic substances, the weak diamagnetic force is overcome by the attractive force of magnetic dipoles in the material. The magnetic permeability of diamagnetic materials is less than the permeability of vacuum, ''μ''0. In most materials, diamagnetism is a weak effect which can be detected only by sensitive laboratory instruments, but a superconductor acts as a strong diamagnet because it repels a magnetic field entirely from its interior. Diamagnetism was first discovered when Anton Brugmans observed in 1778 that bismuth was repel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |