|
Sodium Perrhenate
Sodium perrhenate (also known as sodium rhenate(VII)) is the inorganic compound with the formula NaReO4. It is a white salt that is soluble in water. It is a common precursor to other rhenium compounds. Its structure resembles that of sodium perchlorate and sodium permanganate. Preparation It can be prepared by treatment of rhenium heptoxide with base or by ion exchange Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, ... from the potassium salt. Sodium perrhenate can be prepared from rhenium metal with hydrogen peroxide in the presence of base. :2 Re + 7 H2O2 + 2 NaOH -> 2 NaReO4 + 8 H2O Reactions It reacts with sodium in ethanol to give nonahydridorhenate. Sodium perrhenate has been used as a precursor of rhenium nitrides (such as Re3N, Re2N, Re3N2, ReN2, ReN3, ReN4), which c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food, energy or organic micronutrients. Its chemical formula, H2O, indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. "Water" is also the name of the liquid state of H2O at standard temperature and pressure. A number of natural states of water exist. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice may precipitate in the form of snow. The gaseous state of water is steam or water vapor. Water co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting point of modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one of the rarest elements in the Earth's crust. Rhenium has the third-highest melting point and highest boiling point of any stable element at 5869 K. Rhenium resembles manganese and technetium chemically and is mainly obtained as a by-product of the extraction and refinement of molybdenum and copper ores. Rhenium shows in its compounds a wide variety of oxidation states ranging from −1 to +7. Discovered by Walter Noddack, Ida Tacke and Otto Berg in 1925, rhenium was the last stable element to be discovered. It was named after the river Rhine in Europe, from which the earliest samples had been obtained and worked commercially. Nickel-based superalloys of rhenium are used in combustion chambers, turbine blades, and exhaust nozzles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Perchlorate
Sodium perchlorate is the inorganic compound with the chemical formula Na ClO4. It is a white crystalline, hygroscopic solid that is highly soluble in water and in alcohol. It is usually encountered as the monohydrate. The compound is noteworthy as the most water-soluble of the common perchlorate salts. Sodium perchlorate and other perchlorates has been found on the planet Mars, first detected by the NASA probe Phoenix in 2009. This was later confirmed by spectral analysis by the Mars Reconnaissance Orbiter in 2015 of what is thought to be brine seeps which may be the first evidence of flowing liquid water containing hydrated salts on Mars. Selected properties Its heat of formation is −382.75 kJ/mol, i.e. it is favorable for it to decompose into sodium chloride and dioxygen. It crystallizes in the rhombic crystal system. Uses Sodium perchlorate is the precursor to many other perchlorate salts, often taking advantage of their low solubility relative to NaClO4 (209 g/100 mL at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Permanganate
Sodium permanganate is the inorganic compound with the formula Na MnO4. It is closely related to the more commonly encountered potassium permanganate, but it is generally less desirable, because it is more expensive to produce. It is mainly available as the monohydrate. This salt absorbs water from the atmosphere and has a low melting point. Being about 15 times more soluble than KMnO4, sodium permanganate finds some applications where very high concentrations of MnO4− are sought. Preparation and properties Sodium permanganate cannot be prepared analogously to the route to KMnO4 because the required intermediate manganate salt, Na2MnO4, does not form. Thus less direct routes are used including conversion from KMnO4.Arno H. Reidies "Manganese Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. Sodium permanganate behaves similarly to potassium permanganate. It dissolves readily in water to give deep purple solutions, evaporation of whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhenium Heptoxide
Rhenium(VII) oxide is the inorganic compound with the formula Re2 O7. This yellowish solid is the anhydride of HOReO3. Perrhenic acid, Re2O7·2H2O, is closely related to Re2O7. Re2O7 is the raw material for all rhenium compounds, being the volatile fraction obtained upon roasting the host ore. Structure Solid Re2O7 consists of alternating octahedral and tetrahedral Re centres. Upon heating, the polymer cracks to give molecular (nonpolymeric) Re2O7. This molecular species closely resembles manganese heptoxide, consisting of a pair of ReO4 tetrahedra that share a vertex, i.e., O3Re–O–ReO3. Synthesis and reactions Rhenium(VII) oxide is formed when metallic rhenium or its oxides or sulfides are oxidized at in air. Re2O7 dissolves in water to give perrhenic acid. Heating Re2O7 gives rhenium dioxide, a reaction signalled by the appearance of the dark blue coloration:O. Glemser "Rhenium" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Acade ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion Exchange
Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, the purification of chemicals and separation of substances. Ion exchange usually describes a process of purification of aqueous solutions using solid polymeric ion-exchange resin. More precisely, the term encompasses a large variety of processes where ions are exchanged between two electrolytes. Aside from its use to purify drinking water, the technique is widely applied for purification and separation of a variety of industrially and medicinally important chemicals. Although the term usually refers to applications of synthetic (man-made) resins, it can include many other materials such as soil. Typical ion exchangers are ion-exchange resins (functionalized porous or gel polymer), zeolites, montmorillonite, clay, and soil humus. Ion exc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
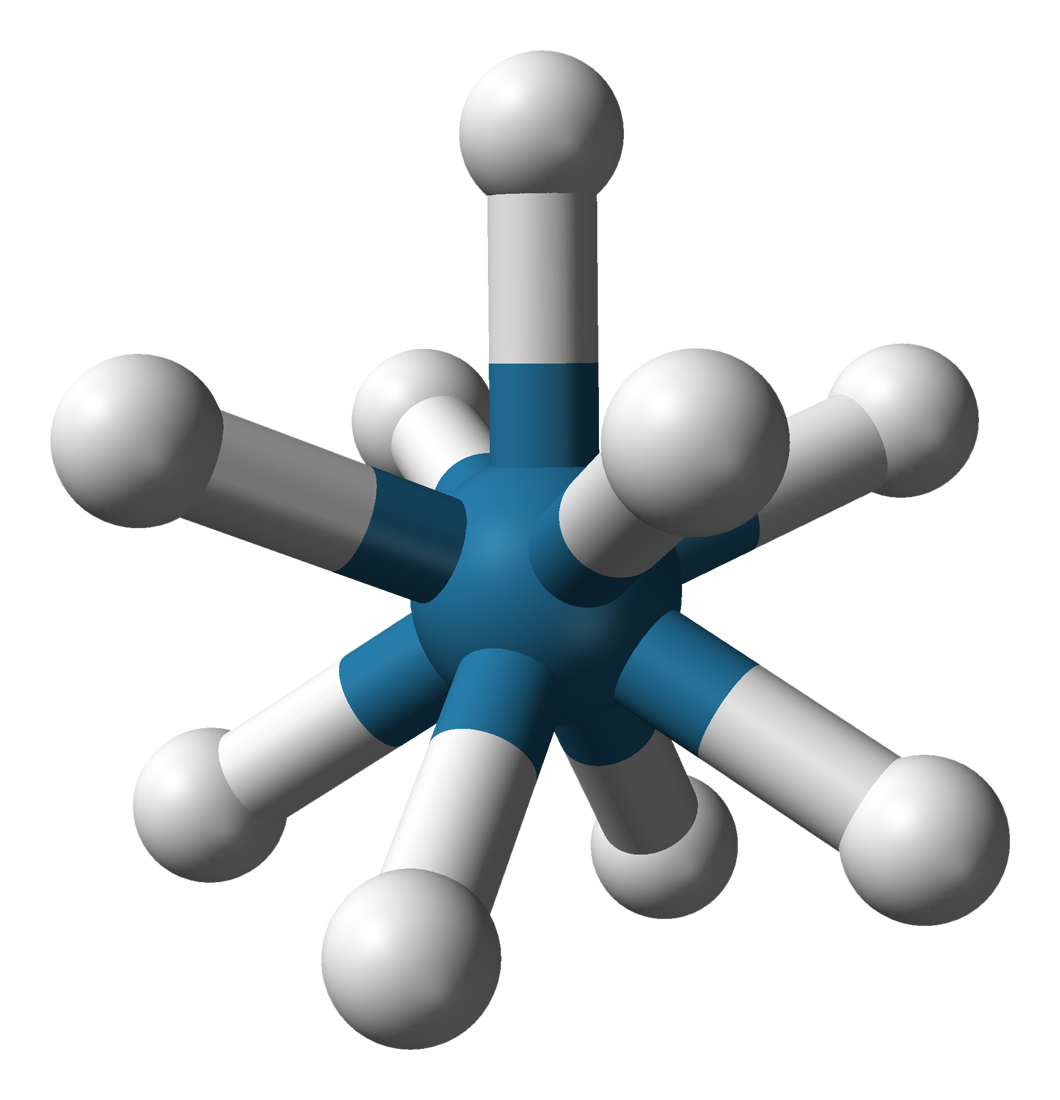
Nonahydridorhenate
Potassium nonahydridorhenate(VII) is an inorganic compound having the formula K2ReH9. This colourless salt is soluble in water but only poorly soluble in most alcohols. The anion is a rare example of a coordination complex bearing only hydride ligands. History The study of rhenium hydrides can be traced to the 1950s and included reports of the "rhenide" anion, supposedly Re−. These reports led to a series of investigations by A. P. Ginsberg and coworkers on the products from the reduction of perrhenate. The ''rhenide'' anion, Re−, was based on the product of the reduction of perrhenate salts, such as the reduction of potassium perrhenate () by potassium metal. "Potassium rhenide" was shown to exist as a tetrahydrated complex, with the postulated chemical formula . This compound exhibits strongly reducing properties, and slowly yields hydrogen gas when dissolved in water. The lithium and thallous salts were also reported. Later research, however, indicates that the "rhenide" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dirhenium Decacarbonyl
Dirhenium decacarbonyl is the inorganic compound with the chemical formula Re2(CO)10 . Commercially available, it is used as a starting point for the synthesis of many rhenium carbonyl complexes. It was first reported in 1941 by Walter Hieber, who prepared it by reductive carbonylation of rhenium. The compound consists of a pair of square pyramidal Re(CO)5 units joined via a Re-Re bond, which produces a homoleptic carbonyl complex. History In the 1930s Robert Mond developed methods which used increased pressure and temperature to produce various forms of metal carbonyl . A prominent scientist of the twentieth century, Walter Hieber was crucial to the further development of specifically the dirhenium decacarbonyl. Initial efforts produced mononuclear metal complexes, but upon further evaluation, Hieber discovered that by using Re2O7 as a starting material with no solvent, a dirhenium complex could be achieved producing a Re-Re interaction. Structure and properties The crystal struc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Compounds
Sodium atoms have 11 electrons, one more than the stable configuration of the noble gas neon. As a result, sodium usually forms ionic compounds involving the Na+ cation. Sodium is a reactive alkali metal and is much more stable in ionic compounds. It can also form intermetallic compounds and organosodium compounds. Sodium compounds are often soluble in water. Metallic sodium Metallic sodium is generally less reactive than potassium and more reactive than lithium. Sodium metal is highly reducing, with the standard reduction potential for the Na+/Na couple being −2.71 volts, though potassium and lithium have even more negative potentials. The thermal, fluidic, chemical, and nuclear properties of molten sodium metal have caused it to be one of the main coolants of choice for the fast breeder reactor. Such nuclear reactors are seen as a crucial step for the production of clean energy. Salts and oxides Sodium compounds are of immense commercial importance, being particularly centra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |