|
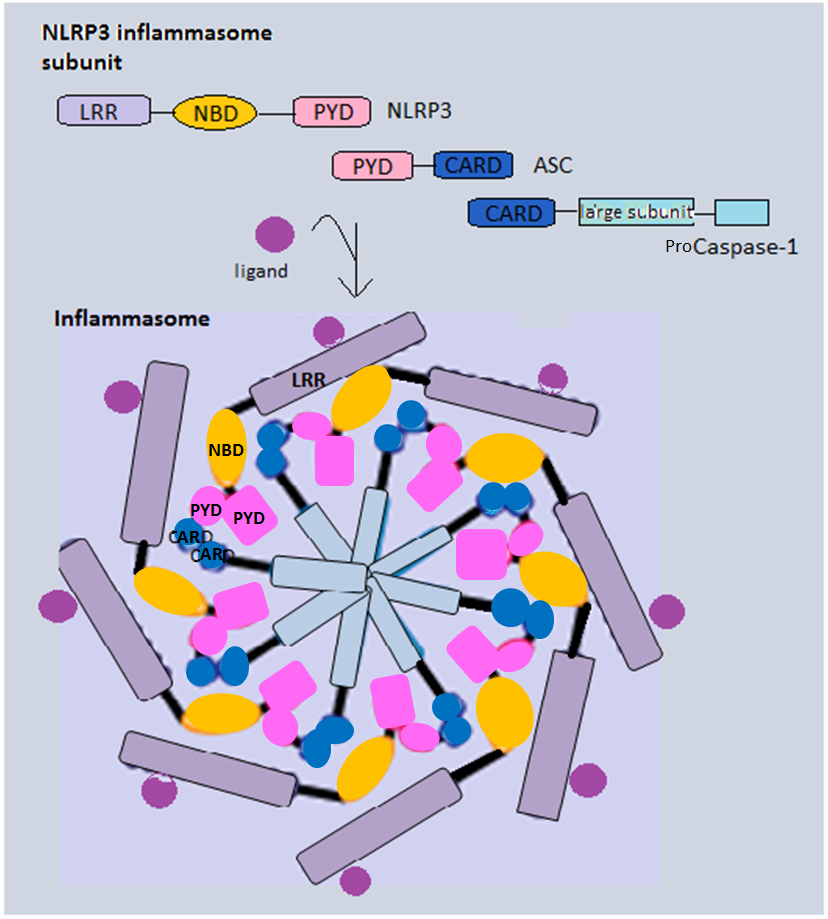
PYCARD
PYCARD, often referred to as ASC (Apoptosis-associated speck-like protein containing a CARD), is a protein that in humans is encoded by the ''PYCARD'' gene. It is localized mainly in the nucleus of monocytes and macrophages. In case of pathogen infection, however, it relocalizes rapidly to the cytoplasm, perinuclear space, endoplasmic reticulum and mitochondria and it is a key adaptor protein in activation of the inflammasome. NMR structure of full-length ASC: PDB ID 2KNref> Function This gene encodes an adaptor protein that is composed of two protein–protein interaction domains: a N-terminus, N-terminal PYRIN-PAAD-DAPIN domain ( PYD) and a C-terminal caspase-recruitment domain (CARD). The PYD and CARD domains are members of the six-helix bundle death domain-fold superfamily that mediates assembly of large signaling complexes in the inflammatory and apoptotic signaling pathways via the activation of caspase. In normal cells, this protein is localized to the cytoplasm; howe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inflammasome
Inflammasomes are cytosolic multiprotein oligomers of the innate immune system responsible for the activation of inflammatory responses. Activation and assembly of the inflammasome promotes proteolytic cleavage, maturation and secretion of pro-inflammatory cytokines interleukin 1β (IL-1β) and interleukin 18 (IL-18), as well as cleavage of Gasdermin-D. The N-terminal fragment resulting from this cleavage induces a pro-inflammatory form of programmed cell death distinct from apoptosis, referred to as pyroptosis, and is responsible for secretion of the mature cytokines, presumably through the formation of pores in the plasma membrane. Inflammasome activation is initiated by different kinds of cytosolic pattern recognition receptors (PRRs) that respond to either microbe-derived pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) generated by the host cell. Pattern recognition receptors involved in inflammasomes comprise NLRs (nucleoti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NOD-like Receptor
The nucleotide-binding oligomerization domain-like receptors, or NOD-like receptors (NLRs) (also known as nucleotide-binding leucine-rich repeat receptors), are intracellular sensors of pathogen-associated molecular patterns (PAMPs) that enter the cell via phagocytosis or pores, and damage-associated molecular patterns (DAMPs) that are associated with cell stress. They are types of pattern recognition receptors (PRRs), and play key roles in the regulation of innate immune response. NLRs can cooperate with toll-like receptors (TLRs) and regulate inflammatory and apoptotic response. They are found in lymphocytes, macrophages, dendritic cells and also in non-immune cells, for example in epithelium. NLRs are highly conserved through evolution. Their homologs have been discovered in many different animal species (APAF1) and also in the plant kingdom ( disease-resistance R protein). Structure NLRs contain 3 domains – central NACHT (NOD or NBD – nucleotide-binding domain) domai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrin Domain
A pyrin domain (PYD, also known as PAAD/DAPIN) is a protein domain and a subclass of protein motif known as the death fold, the 4th and most recently discovered member of the death domain superfamily (DDF). It was originally discovered in the pyrin protein, or marenostrin, encoded by MEFV. The mutation of the MEFV gene is the cause of the disease known as Familial Mediterranean Fever. The domain is encoded in 23 human proteins and at least 31 mouse genes. Proteins containing a pyrin domain are frequently involved in programmed cell death processes including pyroptosis and apoptosis. Proteins that possess a pyrin domain interact with the pyrin domains in other proteins to form of multi-protein complexes called inflammasomes and to trigger downstream immune responses. Structure Pyrin domains are a ~90 amino acid motif present only at the N-terminus of proteins. The core is made of highly conserved hydrophobic residues surrounded by five or six alpha helices with α1→2 linkage ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caspase 1
Caspase-1/Interleukin-1 converting enzyme (ICE) is an evolutionarily conserved enzyme that proteolytically cleaves other proteins, such as the precursors of the inflammatory cytokines interleukin 1β and interleukin 18 as well as the pyroptosis inducer Gasdermin D, into active mature peptides. It plays a central role in cell immunity as an inflammatory response initiator. Once activated through formation of an inflammasome complex, it initiates a proinflammatory response through the cleavage and thus activation of the two inflammatory cytokines, interleukin 1β (IL-1β) and interleukin 18 (IL-18) as well as pyroptosis, a programmed lytic cell death pathway, through cleavage of Gasdermin D. The two inflammatory cytokines activated by Caspase-1 are excreted from the cell to further induce the inflammatory response in neighboring cells. Cellular expression Caspase-1 is evolutionarily conserved in many eukaryotes of the Kingdom Animalia. Due to its role in the inflammatory immune ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CARD Domain
Caspase recruitment domains, or caspase activation and recruitment domains (CARDs), are interaction motifs found in a wide array of proteins, typically those involved in processes relating to inflammation and apoptosis. These domains mediate the formation of larger protein complexes via direct interactions between individual CARDs. CARD domains are found on a strikingly wide range of proteins, including helicases, kinases, mitochondrial proteins, caspases, and other cytoplasmic factors. Basic features CARD domains are a subclass of protein motif known as the death fold, which features an arrangement of six to seven antiparallel alpha helices with a hydrophobic core and an outer face composed of charged residues. Other motifs in this class include the pyrin domain (PYD), death domain (DD), and death effector domain (DED), all of which also function primarily in regulation of apoptosis and inflammatory responses. In apoptosis CARD domains were originally characterized based on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interleukin 1 Beta
Interleukin-1 beta (IL-1β) also known as leukocytic pyrogen, leukocytic endogenous mediator, mononuclear cell factor, lymphocyte activating factor and other names, is a cytokine protein that in humans is encoded by the ''IL1B'' gene."Catabolin" is the name given by Jeremy Saklatvala for IL-1 alpha. There are two genes for interleukin-1 (IL-1): IL-1 alpha and IL-1 beta (this gene). IL-1β precursor is cleaved by cytosolic caspase 1 (interleukin 1 beta convertase) to form mature IL-1β. Function The fever-producing property of human leukocytic pyrogen (interleukin 1) was purified by Dinarello in 1977 with a specific activity of 10–20 nanograms/kg. In 1979, Dinarello reported that purified human leukocytic pyrogen was the same molecule that was described by Igal Gery in 1972. He named it lymphocyte-activating factor (LAF) because it was a lymphocyte mitogen. It was not until 1984 that interleukin 1 was discovered to consist of two distinct proteins, now called interleukin-1 a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Isoform
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene or gene family and are the result of genetic differences. While many perform the same or similar biological roles, some isoforms have unique functions. A set of protein isoforms may be formed from alternative splicings, variable promoter usage, or other post-transcriptional modifications of a single gene; post-translational modifications are generally not considered. (For that, see Proteoforms.) Through RNA splicing mechanisms, mRNA has the ability to select different protein-coding segments ( exons) of a gene, or even different parts of exons from RNA to form different mRNA sequences. Each unique sequence produces a specific form of a protein. The discovery of isoforms could explain the discrepancy between the small number of protein coding regions genes revealed by the human genome project and the large diversity of proteins seen in an organism: different ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caspase
Caspases (cysteine-aspartic proteases, cysteine aspartases or cysteine-dependent aspartate-directed proteases) are a family of protease enzymes playing essential roles in programmed cell death. They are named caspases due to their specific cysteine protease activity – a cysteine in its active site nucleophilically attacks and cleaves a target protein only after an aspartic acid residue. As of 2009, there are 12 confirmed caspases in humans and 10 in mice, carrying out a variety of cellular functions. The role of these enzymes in programmed cell death was first identified in 1993, with their functions in apoptosis well characterised. This is a form of programmed cell death, occurring widely during development, and throughout life to maintain cell homeostasis. Activation of caspases ensures that the cellular components are degraded in a controlled manner, carrying out cell death with minimal effect on surrounding tissues. Caspases have other identified roles in programmed cell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apoptosis
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes (morphology) and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, DNA fragmentation, and mRNA decay. The average adult human loses between 50 and 70 billion cells each day due to apoptosis. For an average human child between eight and fourteen years old, approximately twenty to thirty billion cells die per day. In contrast to necrosis, which is a form of traumatic cell death that results from acute cellular injury, apoptosis is a highly regulated and controlled process that confers advantages during an organism's life cycle. For example, the separation of fingers and toes in a developing human embryo occurs because cells between the digits undergo apoptosis. Unlike necrosis, apoptosis produces cell fragments called apoptotic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene
In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a basic unit of heredity and the molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protein-coding genes and noncoding genes. During gene expression, the DNA is first copied into RNA. The RNA can be directly functional or be the intermediate template for a protein that performs a function. The transmission of genes to an organism's offspring is the basis of the inheritance of phenotypic traits. These genes make up different DNA sequences called genotypes. Genotypes along with environmental and developmental factors determine what the phenotypes will be. Most biological traits are under the influence of polygenes (many different genes) as well as gen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |