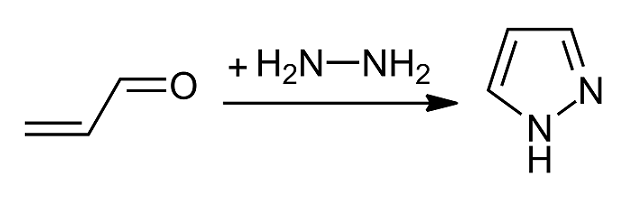|
Pyriprole
Pyriprole (trade name Prac-tic) is for veterinary use on dogs against external parasites such as fleas and ticks. Pyriprole is a phenylpyrazole derivative similar to fipronil. Although recently introduced (in the 2000s) and still under patent protection it is a "classic" insecticide. So far it is only approved in the EU and a few other countries for use on dogs. It is not approved for use on cats or livestock. It has not been introduced as an agricultural or hygiene pesticide. Pyriprole applied as a spot-on is highly effective against fleas and several ticks species. Efficacy against fleas is comparable to that of other modern insecticidal active ingredients such as fipronil, imidacloprid or spinosad. As most flea spot-ons it controls existing flea and tick infestations in about 1 to 2 days, and provides about 4 weeks protection against re-infestations. Mechanism of action Pyriprole is an insecticide and acaricide. It inhibits γ-aminobutyric acid (GABA)-gated chloride channel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flea
Flea, the common name for the order Siphonaptera, includes 2,500 species of small flightless insects that live as external parasites of mammals and birds. Fleas live by ingesting the blood of their hosts. Adult fleas grow to about long, are usually brown, and have bodies that are "flattened" sideways or narrow, enabling them to move through their hosts' fur or feathers. They lack wings; their hind legs are extremely well adapted for jumping. Their claws keep them from being dislodged, and their mouthparts are adapted for piercing skin and sucking blood. They can leap 50 times their body length, a feat second only to jumps made by another group of insects, the superfamily of froghoppers. Flea larvae are worm-like, with no limbs; they have chewing mouthparts and feed on organic debris left on their hosts' skin. Genetic evidence indicates that fleas are a specialised lineage of parasitic scorpionflies (Mecoptera) ''sensu lato'', most closely related to the family Nannochor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flea Allergy Dermatitis
Flea allergy dermatitis is an eczematous itchy skin disease of dogs and cats. For both of these domestic species, flea allergy dermatitis is the most common cause of skin disease. Affected animals develop allergic reactions to chemicals in flea saliva. Symptoms of this reaction include erythema (redness), papules (bumps), pustules (pus-filled bumps), and crusts (scabs). If severe, hair loss will occur in the affected area. Dogs with flea allergy dermatitis often show hair loss and eczematous skin rash on the lower back, upper tail, neck, and down the back of the legs. Cats with flea allergy dermatitis may develop a variety of skin problems, including feline eosinophilic granuloma, miliary dermatitis, or self-inflicted alopecia from excessive grooming. Cause The flea found most commonly on both dogs and cats with a flea infestation is the cat flea, ''Ctenocephalides felis''. Pets that develop flea allergy dermatitis have an allergic response to flea saliva injected during flea feedi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioethers
In organic chemistry, an organic sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A sulfide is similar to an ether except that it contains a sulfur atom in place of the oxygen. The grouping of oxygen and sulfur in the periodic table suggests that the chemical properties of ethers and sulfides are somewhat similar, though the extent to which this is true in practice varies depending on the application. Nomenclature Sulfides are sometimes called thioethers, especially in the old literature. The two organic substituents are indicated by the prefixes. (CH3)2S is called dimethylsulfide. Some sulfides are named by modifying the common name for the corresponding ether. For example, C6H5SCH3 is methyl phenyl sulfide, but is more commonly called thioanisole, since its structure is related to that for anisole, C6H5OCH3. The modern s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
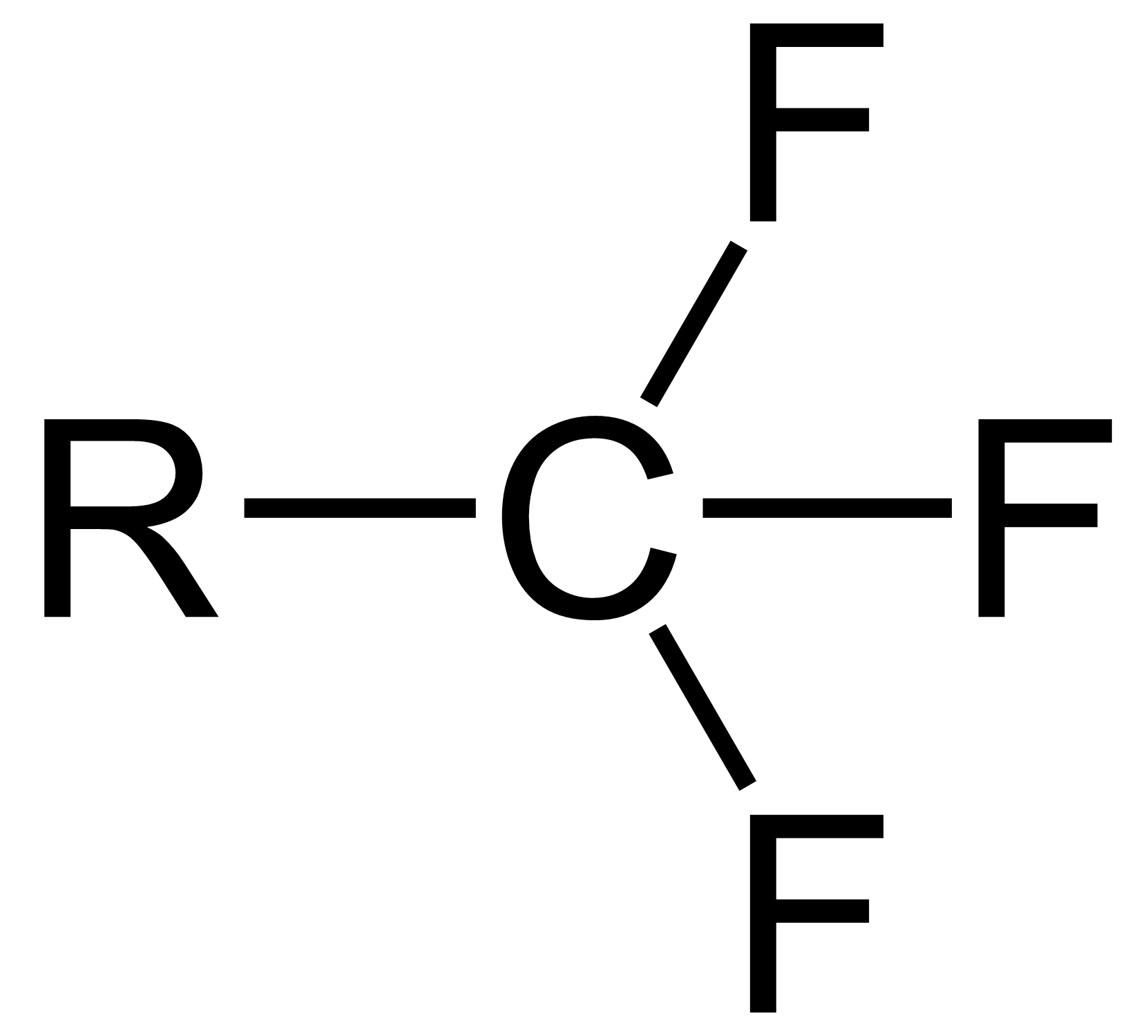
Trifluoromethyl Compounds
The trifluoromethyl group is a functional group that has the formula -CF3. The naming of is group is derived from the methyl group (which has the formula -CH3), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluoromethane H–, 1,1,1-trifluoroethane –, and hexafluoroacetone –CO–. Compounds with this group are a subclass of the organofluorines. Properties The trifluoromethyl group has a significant electronegativity that is often described as being intermediate between the electronegativities of fluorine and chlorine. For this reason, trifluoromethyl-substituted compounds are often strong acids, such as trifluoromethanesulfonic acid and trifluoroacetic acid. Conversely, the trifluoromethyl group lowers the basicity of compounds like trifluoroethanol. Uses The trifluoromethyl group occurs in certain pharmaceuticals, drugs, and abiotically synthesized natural fluorocarbon based compounds. The medicinal use of the trifloromethyl group dates from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organofluorides
Organofluorine chemistry describes the chemistry of the organofluorines, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from Lipophobicity, oil and hydrophobe, water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents. The carbon–fluorine bond Fluorine has several distinctive differences from all other substituents encountered in organic molecules. As a result, the physical and chemical properties of organofluorines can be distinctive in comparison to other organohalogens. # The carbon–fluorine bond is one of the strongest in organic chemistry (an average bond energy around 480 kJ/molKirsch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitriles
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix ''cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons. Inorganic compounds containing the group are not called nitriles, but cyanides instead. Though both nitriles and cyanides can be derived from cyanide salts, most nitriles are not nearly as toxic. Structure and basic properties The N−C−C geometry is linear in nitriles, reflecting the sp hybridization of the triply bonded carbon. The C−N distance is short at 1.16 Å, consistent with a triple bond. Nitriles a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrazoles
Pyrazole is an organic compound with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazole is a weak base, with p''K''b 11.5 (p''K''a of the conjugate acid 2.49 at 25 °C). Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol. Preparation and reactions Pyrazoles are synthesized by the reaction of α,β-unsaturated aldehydes with hydrazine and subsequent dehydrogenation: : Substituted pyrazoles are prepared by condensation of 1,3-diketones with hydrazine ( Knorr-type reactions). For example, acetylacetone and hydrazine gives 3,5-dimethylpyrazole: :CH3C(O)CH2C(O)CH3 + N2H4 → (CH3)2C3HN2H + 2 H2O History The term pyrazole was given to this class of compounds by German ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Insecticides
Insecticides are substances used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. Insecticides are used in agriculture, medicine, industry and by consumers. Insecticides are claimed to be a major factor behind the increase in the 20th-century's agricultural productivity. Nearly all insecticides have the potential to significantly alter ecosystems; many are toxic to humans and/or animals; some become concentrated as they spread along the food chain. Insecticides can be classified into two major groups: systemic insecticides, which have residual or long term activity; and contact insecticides, which have no residual activity. The mode of action describes how the pesticide kills or inactivates a pest. It provides another way of classifying insecticides. Mode of action can be important in understanding whether an insecticide will be toxic to unrelated species, such as fish, birds and mammals. Insecticides may be repellent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GABAA Receptor
The GABAA receptor (GABAAR) is an ionotropic receptor and ligand-gated ion channel. Its endogenous ligand is γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system. Upon opening, the GABAA receptor on the postsynaptic cell is selectively permeable to chloride ions (Cl−) and, to a lesser extent, bicarbonate ions (HCO3−). Depending on the membrane potential and the ionic concentration difference, this can result in ionic fluxes across the pore. If the membrane potential is higher than the equilibrium potential (also known as the reversal potential) for chloride ions, when the receptor is activated Cl− will flow into the cell. This causes an inhibitory effect on neurotransmission by diminishing the chance of a successful action potential occurring at the postsynaptic cell. The reversal potential of the GABAA-mediated inhibitory postsynaptic potential (IPSP) in normal solution is −70 mV, contrasting the GABAB IPSP (-100 mV). T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tick
Ticks (order Ixodida) are parasitic arachnids that are part of the mite superorder Parasitiformes. Adult ticks are approximately 3 to 5 mm in length depending on age, sex, species, and "fullness". Ticks are external parasites, living by feeding on the blood of mammals, birds, and sometimes reptiles and amphibians. The timing of the origin of ticks is uncertain, though the oldest known tick fossils are from the Cretaceous period, around 100 million years old. Ticks are widely distributed around the world, especially in warm, humid climates. Ticks belong to two major families, the Ixodidae or hard ticks, and the Argasidae, or soft ticks. ''Nuttalliella,'' a genus of tick from southern Africa is the only member of the family Nuttalliellidae, and represents the most primitive living lineage of ticks. Adults have ovoid/pear-shaped bodies (idiosomas) which become engorged with blood when they feed, and eight legs. Their cephalothorax and abdomen are completely fused. In addit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |