|
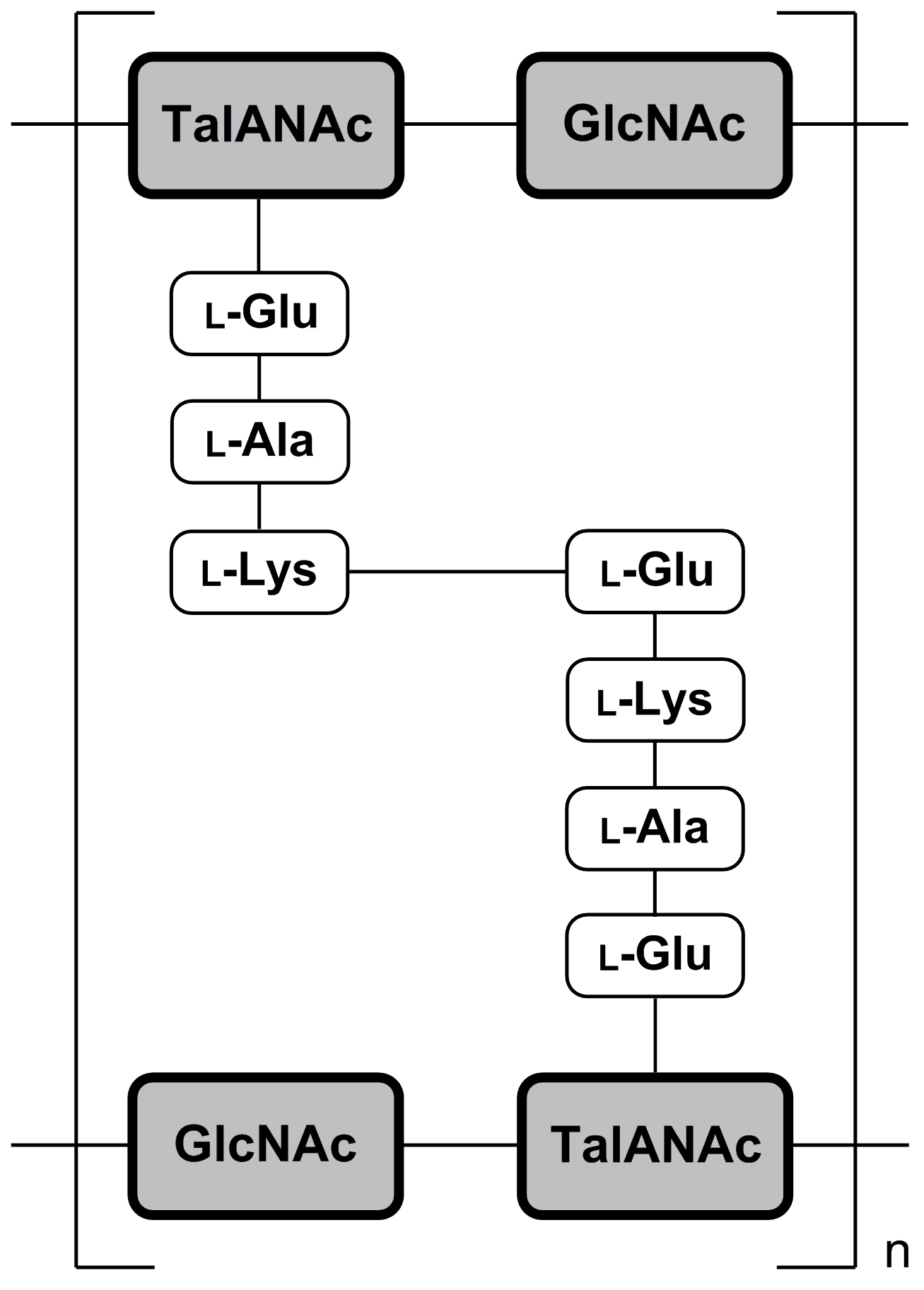
Pseudopeptidoglycan
Pseudopeptidoglycan (also known as pseudomurein;White, David. (1995) ''The Physiology and Biochemistry of Prokaryotes'', pages 6, 12-21. (Oxford: Oxford University Press). . PPG hereafter) is a major cell wall component of some Archaea that differs from bacterial peptidoglycan in chemical structure, but resembles bacterial peptidoglycan in function and physical structure. Pseudopeptidoglycan, in general, is only present in a few methanogenic archaea. The basic components are N-acetylglucosamine and N-acetyltalosaminuronic acid (bacterial peptidoglycan containing ''N''-acetylmuramic acid instead), which are linked by β-1,3-glycosidic bonds. Lysozyme, a host defense mechanism present in human secretions (e.g. saliva and tears) breaks β-1,4-glycosidic bonds to degrade peptidoglycan. However, because pseudopeptidoglycan has β-1,3-glycosidic bonds, lysozyme is ineffective. It was thought from these large differences in cell wall chemistry that archaeal cell walls and bacterial cel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudomurein Endoisopeptidase
Pseudopeptidoglycan (also known as pseudomurein;White, David. (1995) ''The Physiology and Biochemistry of Prokaryotes'', pages 6, 12-21. (Oxford: Oxford University Press). . PPG hereafter) is a major cell wall component of some Archaea that differs from bacterial peptidoglycan in chemical structure, but resembles bacterial peptidoglycan in function and physical structure. Pseudopeptidoglycan, in general, is only present in a few methanogenic archaea. The basic components are N-acetylglucosamine and N-acetyltalosaminuronic acid (bacterial peptidoglycan containing N-Acetylmuramic acid, ''N''-acetylmuramic acid instead), which are linked by β-1,3-glycosidic bonds. Lysozyme, a host defense mechanism present in human secretions (e.g. saliva and tears) breaks β-1,4-glycosidic bonds to degrade peptidoglycan. However, because pseudopeptidoglycan has β-1,3-glycosidic bonds, lysozyme is ineffective. It was thought from these large differences in cell wall chemistry that archaeal cell wall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudopeptidoglycan
Pseudopeptidoglycan (also known as pseudomurein;White, David. (1995) ''The Physiology and Biochemistry of Prokaryotes'', pages 6, 12-21. (Oxford: Oxford University Press). . PPG hereafter) is a major cell wall component of some Archaea that differs from bacterial peptidoglycan in chemical structure, but resembles bacterial peptidoglycan in function and physical structure. Pseudopeptidoglycan, in general, is only present in a few methanogenic archaea. The basic components are N-acetylglucosamine and N-acetyltalosaminuronic acid (bacterial peptidoglycan containing ''N''-acetylmuramic acid instead), which are linked by β-1,3-glycosidic bonds. Lysozyme, a host defense mechanism present in human secretions (e.g. saliva and tears) breaks β-1,4-glycosidic bonds to degrade peptidoglycan. However, because pseudopeptidoglycan has β-1,3-glycosidic bonds, lysozyme is ineffective. It was thought from these large differences in cell wall chemistry that archaeal cell walls and bacterial cel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptidoglycan
Peptidoglycan or murein is a unique large macromolecule, a polysaccharide, consisting of sugars and amino acids that forms a mesh-like peptidoglycan layer outside the plasma membrane, the rigid cell wall (murein sacculus) characteristic of most bacteria (domain ''Bacteria''). The sugar component consists of alternating residues of β-(1,4) linked ''N''-acetylglucosamine (NAG) and ''N''-acetylmuramic acid (NAM). Attached to the ''N''-acetylmuramic acid is a oligopeptide chain made of three to five amino acids. The peptide chain can be cross-linked to the peptide chain of another strand forming the 3D mesh-like layer. Peptidoglycan serves a structural role in the bacterial cell wall, giving structural strength, as well as counteracting the osmotic pressure of the cytoplasm. This repetitive linking results in a dense peptidoglycan layer which is critical for maintaining cell form and withstanding high osmotic pressures, and it is regularly replaced by peptidoglycan production. Pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Archaea
Archaea ( ; singular archaeon ) is a domain of single-celled organisms. These microorganisms lack cell nuclei and are therefore prokaryotes. Archaea were initially classified as bacteria, receiving the name archaebacteria (in the Archaebacteria kingdom), but this term has fallen out of use. Archaeal cells have unique properties separating them from the other two domains, Bacteria and Eukaryota. Archaea are further divided into multiple recognized phyla. Classification is difficult because most have not been isolated in a laboratory and have been detected only by their gene sequences in environmental samples. Archaea and bacteria are generally similar in size and shape, although a few archaea have very different shapes, such as the flat, square cells of ''Haloquadratum walsbyi''. Despite this morphological similarity to bacteria, archaea possess genes and several metabolic pathways that are more closely related to those of eukaryotes, notably for the enzymes involved ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of such infections. They may either kill or inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the common cold or influenza; drugs which inhibit viruses are termed antiviral drugs or antivirals rather than antibiotics. Sometimes, the term ''antibiotic''—literally "opposing life", from the Greek roots ἀντι ''anti'', "against" and βίος ''bios'', "life"—is broadly used to refer to any substance used against microbes, but in the usual medical usage, antibiotics (such as penicillin) are those produced naturally (by one microorganism fighting another), whereas non-antibiotic antibacterials (such as sulfonamides and antiseptics) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flippase
Flippases (rarely spelled flipases) are transmembrane lipid transporter proteins located in the membrane which belong to ABC transporter or P4-type ATPase families. They are responsible for aiding the movement of phospholipid molecules between the two leaflets that compose a cell's membrane (transverse diffusion, also known as a "flip-flop" transition). The possibility of active maintenance of an asymmetric distribution of molecules in the phospholipid bilayer was predicted in the early 1970s by Mark Bretscher. Although phospholipids diffuse rapidly in the plane of the membrane, their polar head groups cannot pass easily through the hydrophobic center of the bilayer, limiting their diffusion in this dimension. Some flippases - often instead called scramblases - are energy-independent and bidirectional, causing reversible equilibration of phospholipid between the two sides of the membrane, whereas others are energy-dependent and unidirectional, using energy from ATP hydrolysis to pu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoglucomutase
Phosphoglucomutase () is an enzyme that transfers a phosphate group on an α-D-glucose monomer from the 1 to the 6 position in the forward direction or the 6 to the 1 position in the reverse direction. More precisely, it facilitates the interconversion of glucose 1-phosphate and glucose 6-phosphate. Biological Function Role in glycogenolysis After glycogen phosphorylase catalyzes the phosphorolytic cleavage of a glucosyl residue from the glycogen polymer, the freed glucose has a phosphate group on its 1-carbon. This glucose 1-phosphate molecule is not itself a useful metabolic intermediate, but phosphoglucomutase catalyzes the conversion of this glucose 1-phosphate to glucose 6-phosphate (see below for the mechanism of this reaction). Glucose 6-phosphate’s metabolic fate depends on the needs of the cell at the time it is generated. If the cell is low on energy, then glucose 6-phosphate will travel down the glycolytic pathway, eventually yielding two molecules of adenosine trip ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Sugar
In organic chemistry, an amino sugar (or more technically a 2-amino-2-deoxysugar) is a sugar molecule in which a hydroxyl group has been replaced with an amine group. More than 60 amino sugars are known, with one of the most abundant being ''N''-Acetyl--glucosamine, which is the main component of chitin. Derivatives of amine containing sugars, such as ''N''-acetylglucosamine and sialic acid, whose nitrogens are part of more complex functional groups rather than formally being amines, are also considered amino sugars. Aminoglycosides are a class of antimicrobial compounds that inhibit bacterial protein synthesis. These compounds are conjugates of amino sugars and aminocyclitols. Synthesis From glycals Glycals are cyclic enol ether derivatives of monosaccharides, having a double bond between carbon atoms 1 and 2 of the ring. ''N''-functionalized of glycals at the C2 position, combined with glycosidic bond formation at C1 is a common strategy for the synthesis of amino sugars. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Penicillin
Penicillins (P, PCN or PEN) are a group of β-lactam antibiotics originally obtained from ''Penicillium'' moulds, principally '' P. chrysogenum'' and '' P. rubens''. Most penicillins in clinical use are synthesised by P. chrysogenum using deep tank fermentation and then purified. A number of natural penicillins have been discovered, but only two purified compounds are in clinical use: penicillin G (intramuscular or intravenous use) and penicillin V (given by mouth). Penicillins were among the first medications to be effective against many bacterial infections caused by staphylococci and streptococci. They are still widely used today for different bacterial infections, though many types of bacteria have developed resistance following extensive use. 10% of the population claims penicillin allergies but because the frequency of positive skin test results decreases by 10% with each year of avoidance, 90% of these patients can tolerate penicillin. Additionally, those with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DD-transpeptidase
DD-transpeptidase (, ''DD-peptidase'', ''DD-transpeptidase'', ''DD-carboxypeptidase'', ''D-alanyl-D-alanine carboxypeptidase'', ''D-alanyl-D-alanine-cleaving-peptidase'', ''D-alanine carboxypeptidase'', ''D-alanyl carboxypeptidase'', and ''serine-type D-Ala-D-Ala carboxypeptidase''.) is a bacterial enzyme that catalyzes the transfer of the R-L-aca-D-alanyl moiety of R-L-aca-D-alanyl-D-alanine carbonyl donors to the γ-OH of their active-site serine and from this to a final acceptor. It is involved in bacterial cell wall biosynthesis, namely, the transpeptidation that crosslinks the peptide side chains of peptidoglycan strands. The antibiotic penicillin irreversibly binds to and inhibits the activity of the transpeptidase enzyme by forming a highly stable penicilloyl-enzyme intermediate. Because of the interaction between penicillin and transpeptidase, this enzyme is also known as penicillin-binding protein (PBP). Mechanism DD-transpeptidase is mechanistically similar to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacterial Infections
Pathogenic bacteria are bacteria that can cause disease. This article focuses on the bacteria that are pathogenic to humans. Most species of bacteria are harmless and are often beneficial but others can cause infectious diseases. The number of these pathogenic species in humans is estimated to be fewer than a hundred. By contrast, several thousand species are part of the gut flora present in the digestive tract. The body is continually exposed to many species of bacteria, including beneficial commensals, which grow on the skin and mucous membranes, and saprophytes, which grow mainly in the soil and in decaying matter. The blood and tissue fluids contain nutrients sufficient to sustain the growth of many bacteria. The body has defence mechanisms that enable it to resist microbial invasion of its tissues and give it a natural immunity or innate resistance against many microorganisms. Pathogenic bacteria are specially adapted and endowed with mechanisms for overcoming the norm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamine
Glutamine (symbol Gln or Q) is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral, polar amino acid. It is non-essential and conditionally essential in humans, meaning the body can usually synthesize sufficient amounts of it, but in some instances of stress, the body's demand for glutamine increases, and glutamine must be obtained from the diet. It is encoded by the codons CAA and CAG. In human blood, glutamine is the most abundant free amino acid. The dietary sources of glutamine include especially the protein-rich foods like beef, chicken, fish, dairy products, eggs, vegetables like beans, beets, cabbage, spinach, carrots, parsley, vegetable juices and also in wheat, papaya, Brussels sprouts, celery, kale and fermented foods like miso. Functions Glutamine plays a role in a variety of biochemical functions: * Pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)