|
Protoxin
Toxication, toxification or toxicity exaltation is the conversion of a chemical compound into a more toxic form in living organisms or in substrates such as soil or water. The conversion can be caused by enzymatic metabolism in the organisms, as well as by abiotic chemical reactions. While the parent drug is usually less active, both the parent drug and its metabolite can be chemically active and cause toxicity, leading to mutagenesis, teratogenesis, and carcinogenesis. Different classes of enzymes, such as P450 monooxygenases, epoxide hydrolase, or acetyltransferases can catalyze the process in the cell, mostly in the liver. Parent non-toxic chemicals are generally referred to as ''protoxins''. While toxication is generally undesirable, in certain cases it is required for the ''in vivo'' conversion of a prodrug to a metabolite with desired pharmacological or toxicological activity. Codeine is an example of a prodrug, metabolized in the body to the active compounds morphine and co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP3A4
Cytochrome P450 3A4 (abbreviated CYP3A4) () is an important enzyme in the body, mainly found in the liver and in the intestine, which in humans is encoded by ''CYP3A4'' gene. It organic redox reaction, oxidizes small foreign organic molecules (xenobiotics), such as toxins or drugs, so that they can be removed from the body. It is highly homologous to CYP3A5, another important CYP3A enzyme. While many drugs are deactivated by CYP3A4, there are also some drugs that are ''activated'' by the enzyme. Some substances, such as some drugs and furanocoumarins present in grapefruit juice, interfere with the action of CYP3A4. These substances will, therefore, either amplify or weaken the action of those drugs that are modified by CYP3A4. CYP3A4 is a member of the cytochrome P450 family of oxidizing enzymes. Several other members of this family are also involved in drug metabolism, but CYP3A4 is the most common and the most versatile one. Like all members of this family, it is a hemoprote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
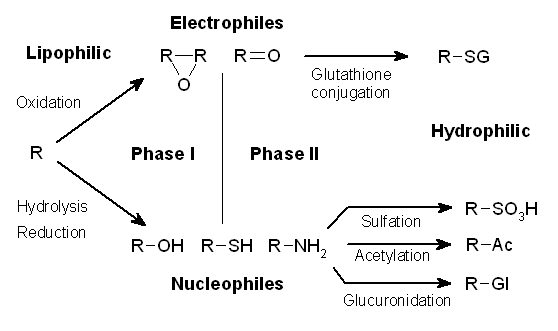
Drug Metabolism
Drug metabolism is the metabolic breakdown of drugs by living organisms, usually through specialized enzymatic systems. More generally, xenobiotic metabolism (from the Greek xenos "stranger" and biotic "related to living beings") is the set of metabolic pathways that modify the chemical structure of xenobiotics, which are compounds foreign to an organism's normal biochemistry, such as any drug or poison. These pathways are a form of biotransformation present in all major groups of organisms and are considered to be of ancient origin. These reactions often act to detoxify poisonous compounds (although in some cases the intermediates in xenobiotic metabolism can themselves cause toxic effects). The study of drug metabolism is the object of pharmacokinetics. Metabolism is one of the stages (see ADME) of the drug's transit through the body that involves the breakdown of the drug so that it can be excreted by the body. The metabolism of pharmaceutical drugs is an important as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Central Nervous System Depression
Central nervous system depression (or CNS depression) is a nervous system disorder characterized by a severely impaired physiological state in which patients may exhibit decreased rate of breathing, decreased heart rate, and loss of consciousness; in extreme cases, CNS depression can possibly lead to coma or death. Causes Central nervous system depression is generally caused by the improper or excessive use of depressant drugs such as opioids, barbiturates, benzodiazepines, general anesthetics, anticonvulsants, and certain sleep medications. These drugs, although useful for treating severe cases of depression that may manifest as CNS depression, can easily be misused. The medications above depress the functions of the spinal cord and brain, both vital components of the central nervous system. In cases of misuse due to addiction, accidents, or unregulated dosage increases, individuals can very easily slip into unconscious coma states because neural activity drops below sa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, Volatility (chemistry), volatile, colorless and flammable liquid with a distinctive alcoholic odor similar to that of ethanol (potable alcohol), but is more acutely toxic than the latter. Methanol acquired the name wood alcohol because it was once produced through destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide. Methanol consists of a methyl group linked to a polar hydroxyl group. With more than 20 million tons produced annually, it is used as a Precursor (chemistry), precursor to other commodity chemicals, including formaldehyde, acetic acid, methyl tert-butyl ether, methyl ''tert''-butyl ether, methyl benzoate, anisole, peroxyacids, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidoreductases
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule, the reductant, also called the electron donor, to another, the oxidant, also called the electron acceptor. This group of enzymes usually utilizes NADP+ or NAD+ as cofactors. Transmembrane oxidoreductases create electron transport chains in bacteria, chloroplasts and mitochondria, including respiratory complexes I, II and III. Some others can associate with biological membranes as peripheral membrane proteins or be anchored to the membranes through a single transmembrane helix. in Membranome database Reactions For exa ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
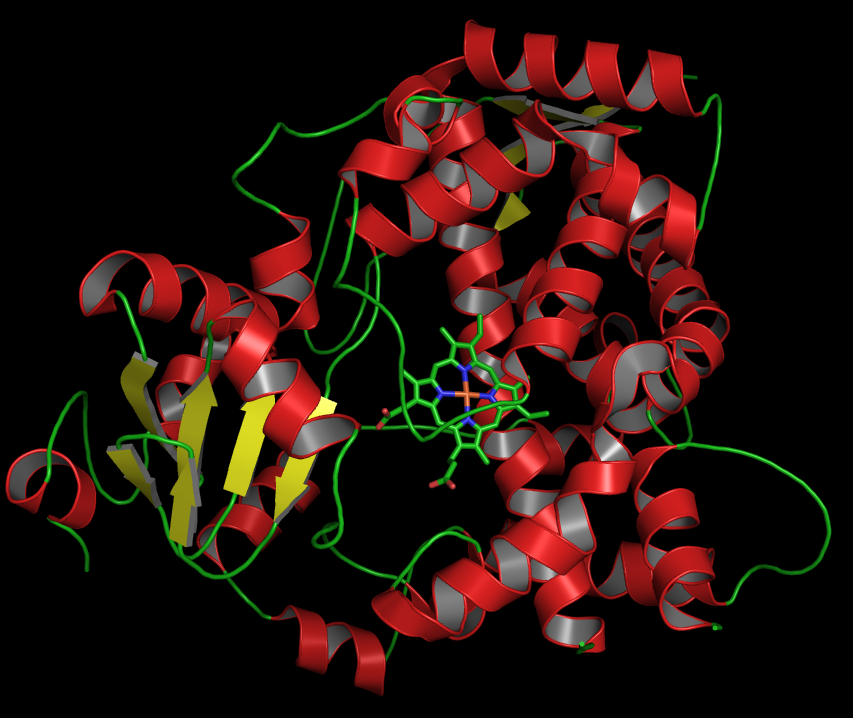
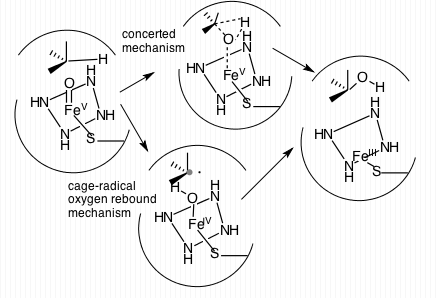
Cytochrome P450 Oxidase
Cytochromes P450 (P450s or CYPs) are a superfamily of enzymes containing heme as a cofactor that mostly, but not exclusively, function as monooxygenases. However, they are not omnipresent; for example, they have not been found in ''Escherichia coli''. In mammals, these enzymes oxidize steroids, fatty acids, xenobiotics, and participate in many biosyntheses. By hydroxylation, CYP450 enzymes convert xenobiotics into hydrophilic derivatives, which are more readily excreted. P450s are, in general, the terminal oxidase enzymes in electron transfer chains, broadly categorized as P450-containing systems. The term "P450" is derived from the spectrophotometric peak at the wavelength of the absorption maximum of the enzyme (450 nm) when it is in the reduced state and complexed with carbon monoxide. Most P450s require a protein partner to deliver one or more electrons to reduce the iron (and eventually molecular oxygen). Nomenclature Genes encoding P450 enzymes, and the e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
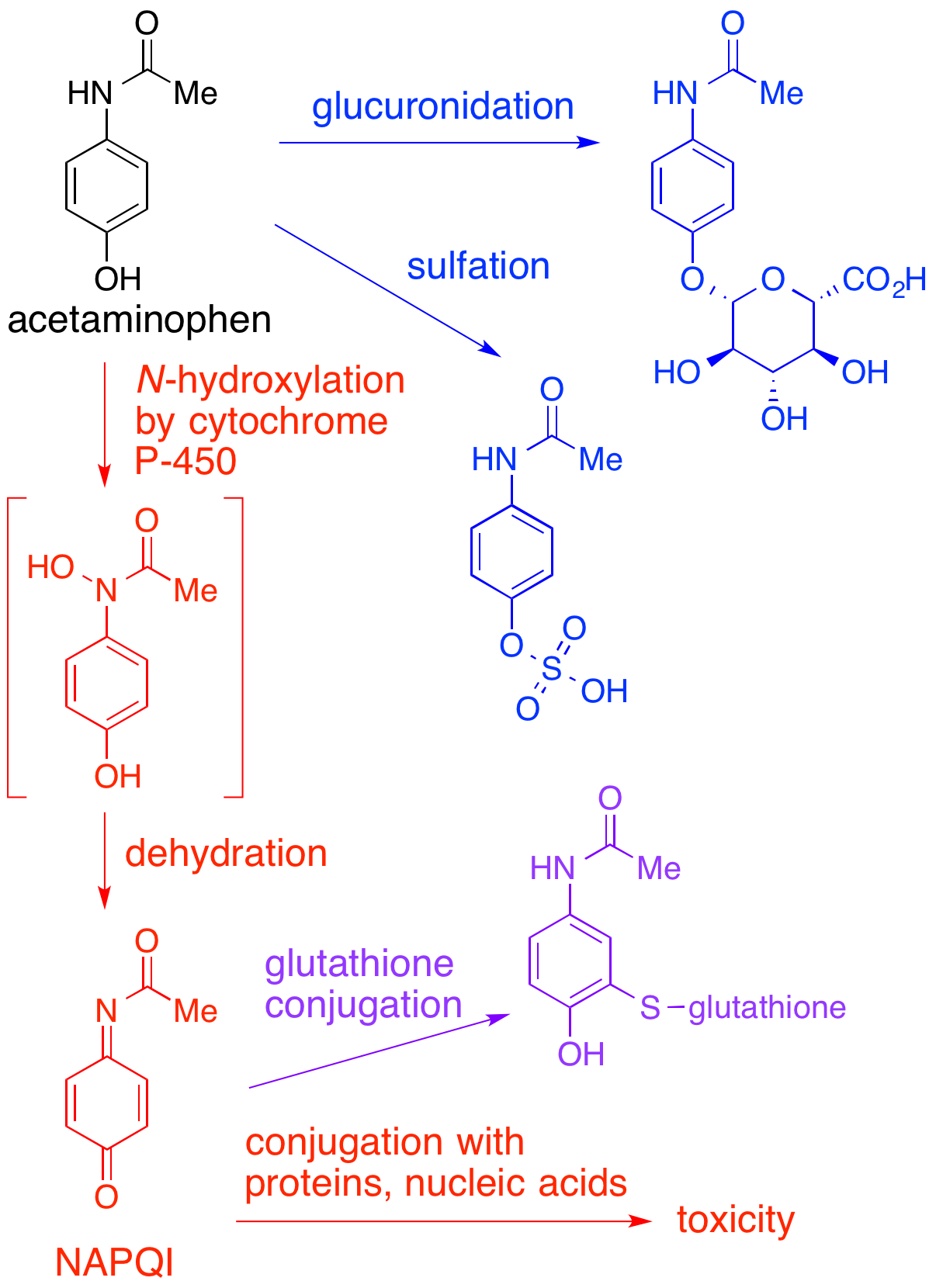
NAPQI
NAPQI, also known as NAPBQI or ''N''-acetyl-''p''-benzoquinone imine, is a toxic byproduct produced during the xenobiotic metabolism of the analgesic paracetamol (acetaminophen). It is normally produced only in small amounts, and then almost immediately detoxified in the liver. However, under some conditions in which NAPQI is not effectively detoxified (usually in the case of paracetamol overdose), it causes severe damage to the liver. This becomes apparent 3–4 days after ingestion and may result in death from fulminant liver failure several days after the overdose. Metabolism In adults, the primary metabolic pathway for paracetamol is glucuronidation. This yields a relatively non-toxic metabolite, which is excreted into bile and passed out of the body. A small amount of the drug is metabolized via the cytochrome P-450 pathway (to be specific, CYP3A4 and CYP2E1) into NAPQI, which is extremely toxic to liver tissue, as well as being a strong biochemical oxidizer. In an av ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paracetamol
Paracetamol, or acetaminophen, is a non-opioid analgesic and antipyretic agent used to treat fever and mild to moderate pain. It is a widely available over-the-counter drug sold under various brand names, including Tylenol and Panadol. Paracetamol relieves pain in both acute mild migraine and episodic tension headache. At a standard dose, paracetamol slightly reduces fever, though it is inferior to ibuprofen in that respect and the benefits of its use for fever are unclear, particularly in the context of fever of viral origins. The aspirin/paracetamol/caffeine combination also helps with both conditions when the pain is mild and is recommended as a Therapy#Lines of therapy, first-line treatment for them. Paracetamol is effective for post-surgical pain, but is inferior to ibuprofen for this purpose. The paracetamol/ibuprofen combination increases the drugs' potency and is superior to either drug alone. The pain relief paracetamol provides in osteoarthritis is small and clinica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Troglitazone
Troglitazone is an antidiabetic and anti-inflammatory drug, and a member of the drug class of the thiazolidinediones. It was prescribed for people with diabetes mellitus type 2. It was patented in 1983 and approved for medical use in 1997. It was subsequently withdrawn. Mechanism of action Troglitazone, like the other thiazolidinediones ( pioglitazone and rosiglitazone), works by activating peroxisome proliferator-activated receptors (PPARs). Troglitazone is a ligand to both PPARα and – more strongly – PPARγ. Troglitazone also contains an α-Tocopherol moiety, potentially giving it vitamin E-like activity in addition to its PPAR activation. It has been shown to reduce inflammation. Troglitazone use was associated with a decrease of nuclear factor kappa-B (NF-κB) and a concomitant increase in its inhibitor (IκB). NFκB is an important cellular transcription regulator for the immune response. History Troglitazone was developed by Daiichi Sankyo (Japan). In t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Troleandomycin
Troleandomycin (TAO for short) is a macrolide antibiotic. It was sold in Italy (branded Triocetin) and Turkey (branded Tekmisin). It is no longer sold in Italy as of 2018. The drug's mode of action is to bind to the ribosome, specifically in the tunnel through which the newly formed peptide egresses, thereby halting protein synthesis. Troleandomycin is a CYP3A4 inhibitor that may cause drug interaction In pharmaceutical sciences, drug interactions occur when a drug's mechanism of action is affected by the concomitant administration of substances such as foods, beverages, or other drugs. A popular example of drug–food interaction is the effect ...s. References CYP3A4 inhibitors Macrolide antibiotics Epoxides Spiro compounds Acetate esters {{antibiotic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flucloxacillin
Flucloxacillin, also known as floxacillin, is an antibiotic used to treat skin infections, external ear infections, infections of leg ulcers, diabetic foot infections, and infection of bone. It may be used together with other medications to treat pneumonia, and endocarditis. It may also be used prior to surgery to prevent ''Staphylococcus'' infections. It is not effective against methicillin-resistant ''Staphylococcus aureus'' (MRSA). It is taken by mouth or given by injection into a vein or muscle. Common side effects include an upset stomach. Other side effects may include muscle or joint pains, shortness of breath, and liver problems. It appears to be safe during pregnancy and breastfeeding. It should not be used in those who are allergic to penicillin. It is a narrow-spectrum beta-lactam antibiotic of the penicillin class. It is similar in effect to cloxacillin and dicloxacillin, being active against penicillinase forming bacteria. Flucloxacillin was patented in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hepatotoxicity
Hepatotoxicity (from ''hepatic toxicity'') implies chemical-driven liver damage. Drug-induced liver injury (DILI) is a cause of acute and chronic liver disease caused specifically by medications and the most common reason for a drug to be withdrawn from the market after approval. The liver plays a central role in transforming and clearing chemicals and is susceptible to the toxicity from these agents. Certain medicinal agents, when taken in overdoses (e.g. acetaminophen, paracetamol) and sometimes even when introduced within therapeutic ranges (e.g. halothane), may injure the organ. Other chemical agents, such as those used in laboratories and industries, natural chemicals (e.g., alpha-amanitin), and herbal remedies (two prominent examples being kava, though the causal mechanism is unknown, and comfrey, through pyrrolizidine alkaloid content) can also induce hepatotoxicity. Chemicals that cause liver injury are called hepatotoxins. More than 900 drugs have been implicated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |