|
Protactinium(IV) Oxide
Protactinium(IV) oxide is a chemical compound with the formula Pa O2. The black oxide is formed by reducing Pa2O5 with hydrogen at 1 550 °C. Protactinium(IV) oxide does not dissolve in H2SO4, HNO3, or HCl solutions, but reacts with HF.Boris F. Myasoedov, H. W. Kirby, & Ivan G. Tananaev (2006) Protactinium, Chapter 4 in Morss, Lester R. & Edelstein, Norman M. & Fuger, Jean, (edit.The Chemistry of the Actinide and Transactinide Elements(PDF) (3. painos). Dordrecht: Springer. ss. 161–252. As protactinium(IV) oxide, like other protactinium compounds, is radioactive Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ..., toxic and very rare, it has no known technological use. References Oxides Protactinium compounds Fluorite crystal structure {{Inorganic-compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
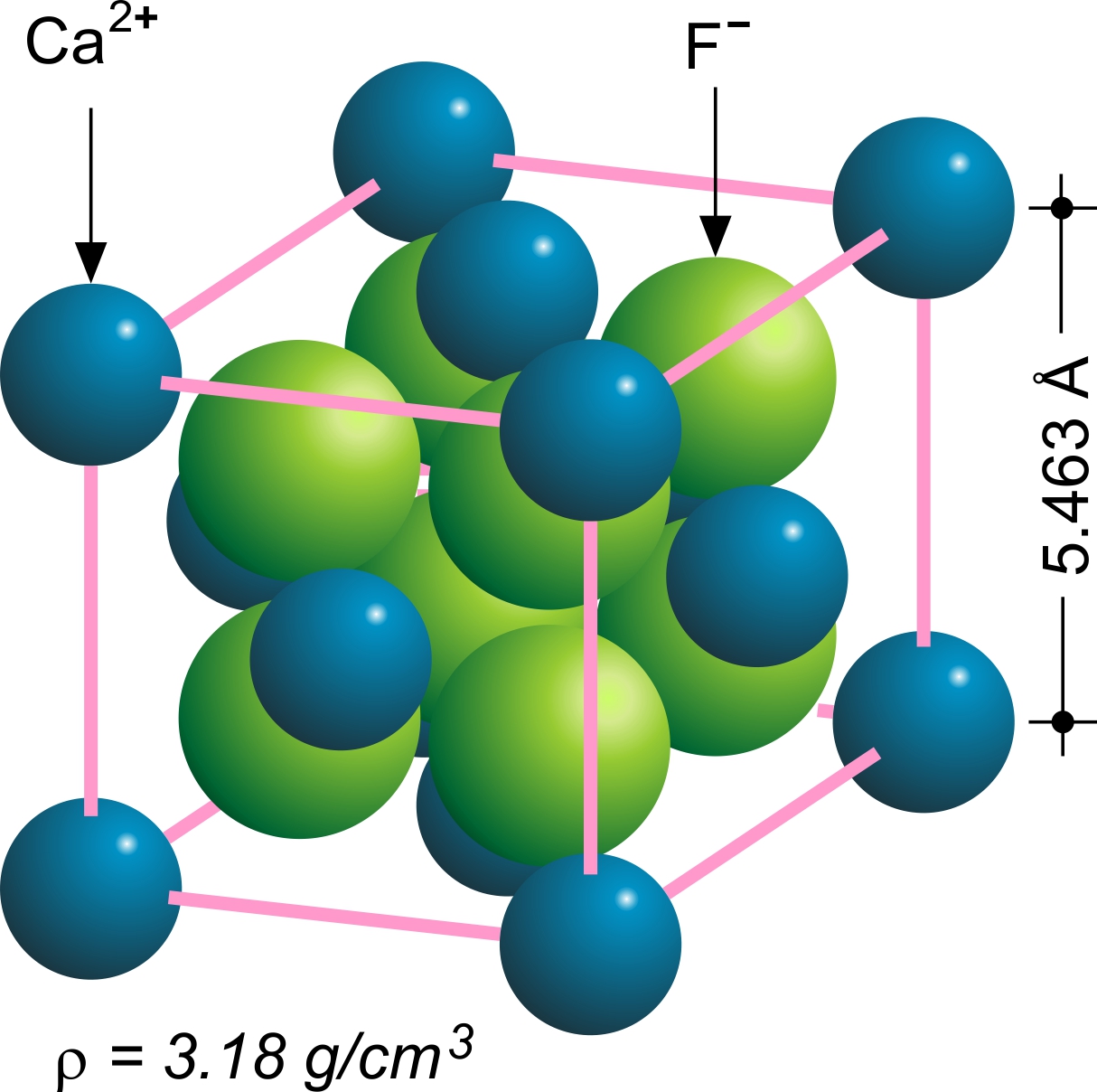
Fluorite
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon. The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 4 as fluorite. Pure fluorite is colourless and transparent, both in visible and ultraviolet light, but impurities usually make it a colorful mineral and the stone has ornamental and lapidary uses. Industrially, fluorite is used as a flux for smelting, and in the production of certain glasses and enamels. The purest grades of fluorite are a source of fluoride for hydrofluoric acid manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite lenses have low dispersion, so lenses made from it exhibit less chromatic aberration, making them valuable in microscopes and telescopes. Fluorite optics are also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pearson Symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure, and was originated by W. B. Pearson. The symbol is made up of two letters followed by a number. For example: * Diamond structure, ''cF''8 * Rutile structure, ''tP''6 The two (italicised) letters specify the Bravais lattice. The lower-case letter specifies the crystal family, and the upper-case letter the centering type. The number at the end of the Pearson symbol gives the number of the atoms in the conventional unit cell.Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005 IR-3.4.4, pp. 49–51; IR-11.5, pp. 241–242. |
Protactinium
Protactinium (formerly protoactinium) is a chemical element with the symbol Pa and atomic number 91. It is a dense, silvery-gray actinide metal which readily reacts with oxygen, water vapor and inorganic acids. It forms various chemical compounds in which protactinium is usually present in the oxidation state +5, but it can also assume +4 and even +3 or +2 states. Concentrations of protactinium in the Earth's crust are typically a few parts per trillion, but may reach up to a few parts per million in some uraninite ore deposits. Because of its scarcity, high radioactivity and high toxicity, there are currently no uses for protactinium outside scientific research, and for this purpose, protactinium is mostly extracted from spent nuclear fuel. The element was first identified in 1913 by Kazimierz Fajans and Oswald Helmuth Göhring and named ''brevium'' because of the short half-life of the specific isotope studied, i.e. protactinium-234. A more stable isotope of protactinium, 231Pa, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats, as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protactinium(V) Oxide
Protactinium(V) oxide is a chemical compound with the formula Pa2 O5. When it is reduced with hydrogen, it forms PaO2. Aristid V. Grosse was first to prepare 2 mg of Pa2O5 in 1927. Pa2O5 does not dissolve in concentrated HNO3, but dissolves in HF and in a HF + H2SO4 mixture and reacts at high temperatures with solid oxides of alkali metal and alkaline earth metal The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar properties: they are all ...s.Boris F. Myasoedov, H. W. Kirby, & Ivan G. Tananaev (2006) Protactinium, Chapter 4 in Morss, Lester R. & Edelstein, Norman M. & Fuger, Jean, (edit.The Chemistry of the Actinide and Transactinide Elements(PDF) (3. painos). Dordrecht: Springer. ss. 161–252. As protactinium(V) oxide, like other protactinium compounds, is radioactive, toxic and very rare, it has ve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered radioactive. Three of the most common types of decay are alpha decay ( ), beta decay ( ), and gamma decay ( ), all of which involve emitting one or more particles. The weak force is the mechanism that is responsible for beta decay, while the other two are governed by the electromagnetism and nuclear force. A fourth type of common decay is electron capture, in which an unstable nucleus captures an inner electron from one of the electron shells. The loss of that electron from the shell results in a cascade of electrons dropping down to that lower shell resulting in emission of discrete X-rays from the transitions. A common example is iodine-125 commonly used in medical settings. Radioactive decay is a stochastic (i.e. random) process ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxides
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 (called a passivation layer) that protects the foil from further corrosion.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. . Stoichiometry (the measurable relationship between reactants and chemical equations of a equation or reaction) Oxides are extraordinarily diverse in terms of stoichiometries and in terms of the structures of each stoichiometry. Most elements form oxides of more than one stoichiometry. A well known example is carbon monoxide and carbon dioxide.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protactinium Compounds
Protactinium compounds are compounds containing the element protactinium (symbol: Pa). These compounds usually have protactinium in the +5 oxidation state, although these compounds can also exist in the +2, +3 and +4 oxidation states. Properties of protactinium compounds Here ''a'', ''b'' and ''c'' are lattice constants in picometers, No is space group number and ''Z'' is the number of formula units per unit cell; ''fcc'' stands for the face-centered cubic symmetry. Density was not measured directly but calculated from the lattice parameters. Oxides and oxygen-containing salts Protactinium oxides are known for the metal oxidation states +2, +4 and +5. The most stable is white pentoxide Pa2O5, which can be produced by igniting protactinium(V) hydroxide in air at a temperature of 500 °C. Greenwood, p. 1268 Its crystal structure is cubic, and the chemical composition is often non-stoichiometric, described as PaO2.25. Another phase of this oxide with orthorhombic symmet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |