|
Promethium(III) Oxide
Promethium(III) oxide is a compound with the formula Pm2O3. It is the most common form of promethium Promethium is a chemical element with the symbol Pm and atomic number 61. All of its isotopes are radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in Earth's crust at any given time. Promethium is one of onl .... Crystal structure Promethium oxide exists in three major crystalline forms: *a, b and c are lattice parameters, Z is the number of formula units per unit cell, density is calculated from X-ray data. The low-temperature cubic form converts to the monoclinic structure upon heating to 750–800 °C, and this transition can only be reversed by melting the oxide. The transition from the monoclinic to hexagonal form occurs at 1740 °C. References Promethium compounds Sesquioxides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Promethium(III) Chloride
Promethium(III) chloride is a chemical compound of promethium and chlorine with the formula PmCl3. It is an ionic, water soluble, crystalline salt that glows in the dark with a pale blue or green light due to promethium's intense radioactivity. Applications Promethium(III) chloride (with 147Pm) has been used to generate long-lasting glow in signal lights and buttons. This application relied on the unstable nature of promethium, which emitted beta radiation (electrons) with a half-life of several years. The electrons were absorbed by a phosphor A phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescent substances which glow on exposure to ultraviolet or vi ..., generating visible glow. Unlike many other radioactive nuclides, promethium-147 does not emit alpha particles that would degrade the phosphor. References Promethium compounds Chlorid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium(III) Oxide
Neodymium(III) oxide or neodymium sesquioxide is the chemical compound composed of neodymium and oxygen with the formula Nd2O3. It forms very light grayish-blue hexagonal crystals. The rare-earth mixture didymium, previously believed to be an element, partially consists of neodymium(III) oxide. Uses Neodymium(III) oxide is used to dope glass, including sunglasses, to make solid-state lasers, and to color glasses and enamels. Neodymium-doped glass turns purple due to the absorbance of yellow and green light, and is used in welding goggles. Some neodymium-doped glass is dichroic; that is, it changes color depending on the lighting. One kind of glass named for the mineral alexandrite appears blue in sunlight and red in artificial light. About 7000 tonnes of neodymium(III) oxide are produced worldwide each year. Neodymium(III) oxide is also used as a polymerization catalyst. Reactions Neodymium(III) oxide is formed when neodymium(III) nitride or neodymium(III) hydroxide is roas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
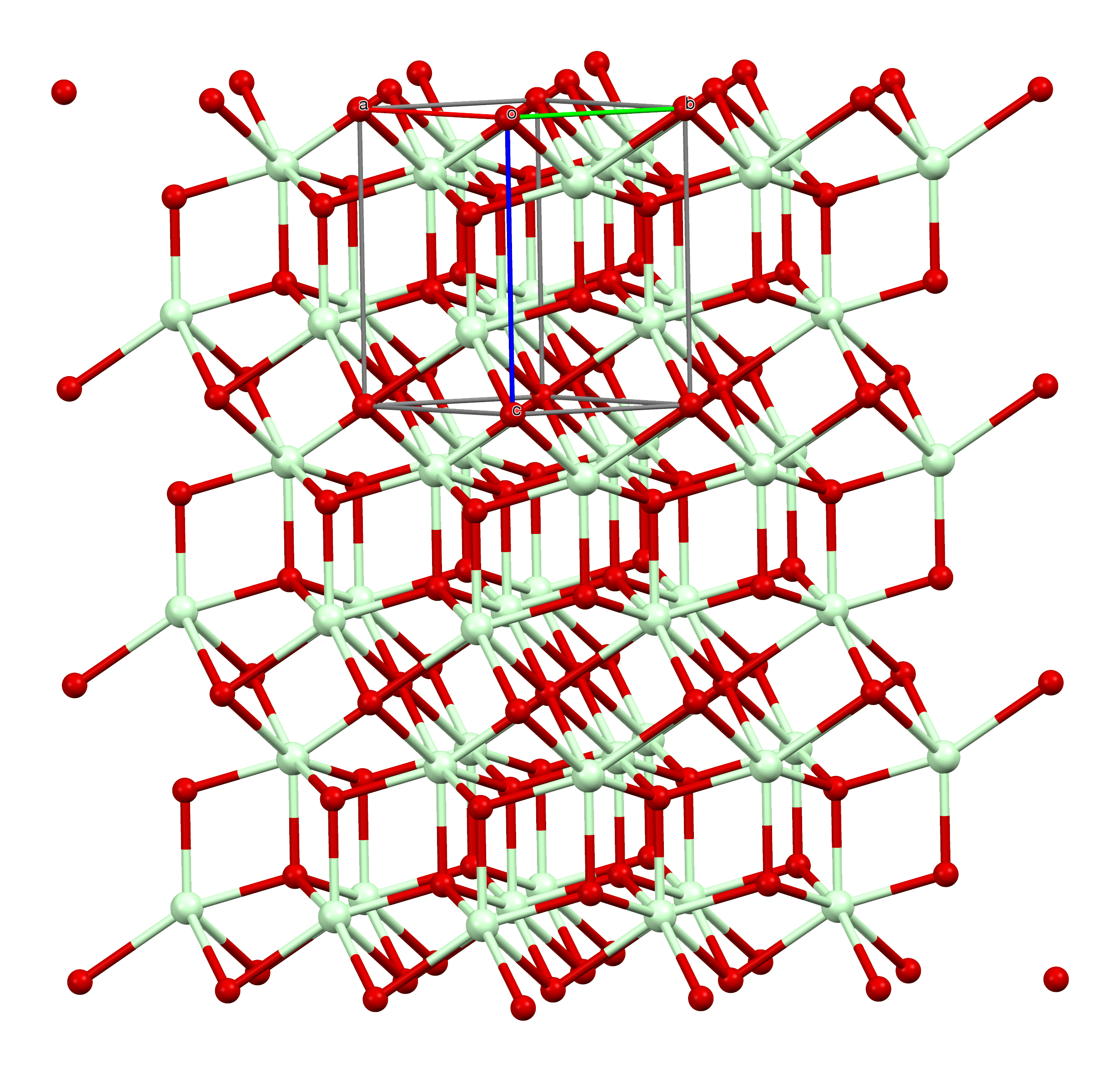
Samarium(III) Oxide
Samarium(III) oxide ( Sm2 O3) is a chemical compound. Samarium oxide readily forms on the surface of samarium metal under humid conditions or temperatures in excess of 150°C in dry air. Similar to rust on metallic iron, this oxide layer spalls off the surface of the metal, exposing more metal to continue the reaction. The oxide is commonly white to off yellow in color and is often encountered as a highly fine dust like powder. Uses Samarium(III) oxide is used in optical and infrared absorbing glass to absorb infrared radiation. Also, it is used as a neutron absorber in control rods for nuclear power reactors. The oxide catalyzes the dehydration and dehydrogenation of primary and secondary alcohols. Another use involves preparation of other samarium salts. Pradyot Patnaik. ''Handbook of Inorganic Chemicals''. McGraw-Hill, 2002, Preparations Samarium(III) oxide may be prepared by two methods: 1. thermal decomposition of samarium(III) carbonate, hydroxide, nitrate, oxalate or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neptunium(III) Oxide
Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive actinide metal, neptunium is the first transuranic element. Its position in the periodic table just after uranium, named after the planet Uranus, led to it being named after Neptune, the next planet beyond Uranus. A neptunium atom has 93 protons and 93 electrons, of which seven are valence electrons. Neptunium metal is silvery and tarnishes when exposed to air. The element occurs in three allotropic forms and it normally exhibits five oxidation states, ranging from +3 to +7. It is radioactive, poisonous, pyrophoric, and capable of accumulating in bones, which makes the handling of neptunium dangerous. Although many false claims of its discovery were made over the years, the element was first synthesized by Edwin McMillan and Philip H. Abelson at the Berkeley Radiation Laboratory in 1940. Since then, most neptunium has been and still is produced by neutron irradiation of uranium in nuclear re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Promethium
Promethium is a chemical element with the symbol Pm and atomic number 61. All of its isotopes are radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in Earth's crust at any given time. Promethium is one of only two radioactive elements that are followed in the periodic table by elements with stable forms, the other being technetium. Chemically, promethium is a lanthanide. Promethium shows only one stable oxidation state of +3. In 1902 Bohuslav Brauner suggested that there was a then-unknown element with properties intermediate between those of the known elements neodymium (60) and samarium (62); this was confirmed in 1914 by Henry Moseley, who, having measured the atomic numbers of all the elements then known, found that atomic number 61 was missing. In 1926, two groups (one Italian and one American) claimed to have isolated a sample of element 61; both "discoveries" were soon proven to be false. In 1938, during a nuclear experiment conducted a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pearson Symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure, and was originated by W. B. Pearson. The symbol is made up of two letters followed by a number. For example: * Diamond structure, ''cF''8 * Rutile structure, ''tP''6 The two (italicised) letters specify the Bravais lattice. The lower-case letter specifies the crystal family, and the upper-case letter the centering type. The number at the end of the Pearson symbol gives the number of the atoms in the conventional unit cell.Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005 IR-3.4.4, pp. 49–51; IR-11.5, pp. 241–242. |
Space Group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it unchanged. In three dimensions, space groups are classified into 219 distinct types, or 230 types if chiral copies are considered distinct. Space groups are discrete cocompact groups of isometries of an oriented Euclidean space in any number of dimensions. In dimensions other than 3, they are sometimes called Bieberbach groups. In crystallography, space groups are also called the crystallographic or Fedorov groups, and represent a description of the symmetry of the crystal. A definitive source regarding 3-dimensional space groups is the ''International Tables for Crystallography'' . History Space groups in 2 dimensions are the 17 wallpaper groups which have been known for several centuries, though the proof that the list was complete was only ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Promethium Compounds
Promethium compounds are compounds containing the element promethium, which normally take the +3 oxidation state. Promethium belongs to the cerium group of lanthanides and is chemically very similar to the neighboring elements. Because of its instability, chemical studies of promethium are incomplete. Even though a few compounds have been synthesized, they are not fully studied; in general, they tend to be pink or red in color.promethium Encyclopædia Britannica Online Treatment of acidic solutions containing Pm3+ ions with ammonia results in a gelatinous light-brown sediment of hydroxide, Pm(OH)3, which is insoluble in water. When dissolved in hydrochloric acid, a water-soluble yellow salt, PmCl3, is produced; simila ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.png)
