|
Process Safety
Process safety focuses on preventing fires, explosions and chemical accidents in chemical process facilities or other facilities dealing with hazardous materials such as refineries, and oil and gas (onshore and offshore) production installations. Occupational safety and health primarily covers the management of personal safety. Well developed management systems also address process safety issues. The tools, techniques, programs etc. required to manage both process and occupational safety can sometimes be the same (for example a work permit system) and in other cases may have very different approaches. LOPA ( Layers of Protection Analysis) or QRA ( Quantified Risk Assessment) for example focus on process safety whereas PPE (Personal Protective Equipment) is very much an individual focused occupational safety issue. A common tool used to explain the various different but connected systems related to achieving process safety is described by James T. Reason's Swiss cheese model. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fire
Fire is the rapid oxidation of a material (the fuel) in the exothermic chemical process of combustion, releasing heat, light, and various reaction Product (chemistry), products. At a certain point in the combustion reaction, called the ignition point, flames are produced. The ''flame'' is the visible portion of the fire. Flames consist primarily of carbon dioxide, water vapor, oxygen and nitrogen. If hot enough, the gases may become ionized to produce Plasma (physics), plasma. Depending on the substances alight, and any impurities outside, the color of the flame and the fire's Intensity (heat transfer), intensity will be different. Fire in its most common form can result in conflagration, which has the potential to cause physical damage through burning. Fire is an important process that affects ecological systems around the globe. The positive effects of fire include stimulating growth and maintaining various ecological systems. Its negative effects include hazard to life and pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Swiss Cheese Model
The Swiss cheese model of accident causation is a model used in risk analysis and risk management, including aviation safety, engineering, healthcare, emergency service organizations, and as the principle behind layered security, as used in computer security and defense in depth. It likens human systems to multiple slices of Swiss cheese, stacked side by side, in which the risk of a threat becoming a reality is mitigated by the differing layers and types of defenses which are "layered" behind each other. Therefore, in theory, lapses and weaknesses in one defense do not allow a risk to materialize, since other defenses also exist, to prevent a single point of failure. The model was originally formally propounded by James T. Reason of the University of Manchester, and has since gained widespread acceptance. It is sometimes called the "cumulative act effect". Although the Swiss cheese model is respected and considered to be a useful method of relating concepts, it has been subj ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Safety
Functional safety is the part of the overall safety of a system or piece of equipment that depends on automatic protection operating correctly in response to its inputs or failure in a predictable manner (fail-safe). The automatic protection system should be designed to properly handle likely human errors, systematic errors, hardware failures and operational/environmental stress. Objective The objective of functional safety is freedom from unacceptable risk of physical injury or of damage to the health of people either directly or indirectly (through damage to property or to the environment) by the proper implementation of one or more automatic protection functions (often called safety functions). A safety system (often called a safety-related system) consists of one or more safety functions. Functional safety is intrinsically end-to-end in scope in that it has to treat the function of a component or subsystem as part of the function of the entire automatic protection function ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
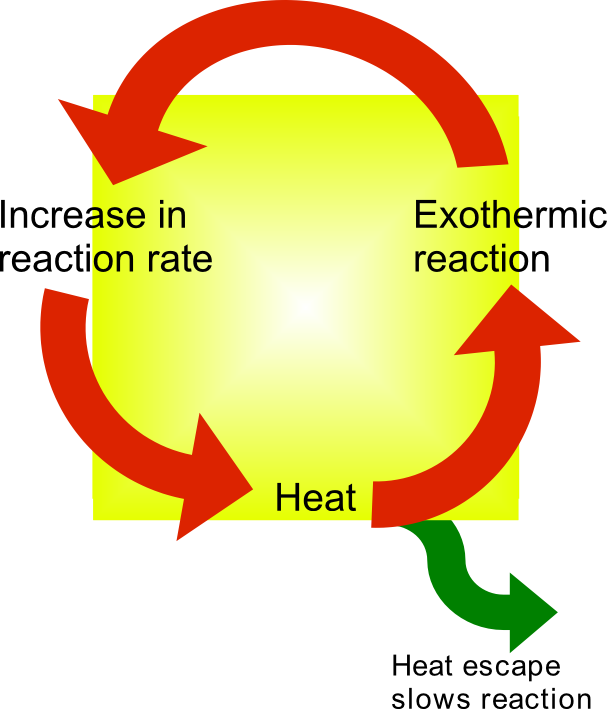
Thermal Runaway
Thermal runaway describes a process that is accelerated by increased temperature, in turn releasing energy that further increases temperature. Thermal runaway occurs in situations where an increase in temperature changes the conditions in a way that causes a further increase in temperature, often leading to a destructive result. It is a kind of uncontrolled positive feedback. In chemistry (and chemical engineering), thermal runaway is associated with strongly exothermic reactions that are accelerated by temperature rise. In electrical engineering, thermal runaway is typically associated with increased current flow and power dissipation. Thermal runaway can occur in civil engineering, notably when the heat released by large amounts of curing concrete is not controlled. In astrophysics, runaway nuclear fusion reactions in stars can lead to nova and several types of supernova explosions, and also occur as a less dramatic event in the normal evolution of solar-mass stars, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Distillation
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the heating of solid materials to produce gaseous products (which may condense into liquids or solids); this may involve chemical changes such as destructive distillation or cracking. Distillation may result in essentially complete separation (resulting in nearly pure components), or it may be a partial separation that increases the concentration of selected components; in either case, the process exploits differences in the relative volatility of the mixture's components. In industrial applications, distillation is a unit operation of practically universal importance, but is a physical separation process, not a chemical reaction. An installation used for distillation, especially of distilled beverages, is a distillery. Distillation include ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calorimeter
A calorimeter is an object used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity. Differential scanning calorimeters, isothermal micro calorimeters, titration calorimeters and accelerated rate calorimeters are among the most common types. A simple calorimeter just consists of a thermometer attached to a metal container full of water suspended above a combustion chamber. It is one of the measurement devices used in the study of thermodynamics, chemistry, and biochemistry. To find the enthalpy change per mole of a substance A in a reaction between two substances A and B, the substances are separately added to a calorimeter and the initial and final temperatures (before the reaction has started and after it has finished) are noted. Multiplying the temperature change by the mass and specific heat capacities of the substances gives a value for the energy given off or absorbed during the reaction. Dividing t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactive Screening Device
{{disambig ...
Reactive may refer to: *Generally, capable of having a reaction (other) *An adjective abbreviation denoting a bowling ball coverstock made of reactive resin *Reactivity (chemistry) *Reactive mind *Reactive programming See also * Reactance (other) *Reactivity (other) Reactivity may refer to: * Reactivity (chemistry), the rate at which a chemical substance tends to undergo a chemical reaction * Reactive programming, a property of an execution model whereby changes are automatically propagated through a dataf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Differential Scanning Calorimeter
Differential scanning calorimetry (DSC) is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. Both the sample and reference are maintained at nearly the same temperature throughout the experiment. Generally, the temperature program for a DSC analysis is designed such that the sample holder temperature increases linearly as a function of time. The reference sample should have a well-defined heat capacity over the range of temperatures to be scanned. The technique was developed by E. S. Watson and M. J. O'Neill in 1962, and introduced commercially at the 1963 Pittsburgh Conference on Analytical Chemistry and Applied Spectroscopy. The first adiabatic differential scanning calorimeter that could be used in biochemistry was developed by P. L. Privalov and D. R. Monaselidze in 1964 at Institute of Physics in Tbilisi, Georgia. The term DSC was coined to descr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Calorimeter
A reaction calorimeter is a calorimeter that measures the amount of energy released (exothermic) or absorbed (endothermic) by a chemical reaction. These measurements provide a more accurate picture of such reactions. Applications When considering scaling up a reaction to large scale from lab scale, it is important to understand how much heat is released. At a small scale, heat released may not cause a concern, however when scaling up, build up can be extremely dangerous. Crystallizing a reaction product from solution is a highly cost effective purification technique. It is therefore valuable to be able to measure how effectively crystallization is taking place in order to be able to optimize it. The heat absorbed by the process can be a useful measure. The energy being released by any process in the form of heat is directly proportional to the rate of reaction and hence reaction calorimetry (as a time resolved measurement technique) can be used to study kinetics. The use of r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fatigue (material)
In materials science, fatigue is the initiation and propagation of cracks in a material due to cyclic loading. Once a fatigue crack has initiated, it grows a small amount with each loading cycle, typically producing striations on some parts of the fracture surface. The crack will continue to grow until it reaches a critical size, which occurs when the stress intensity factor of the crack exceeds the fracture toughness of the material, producing rapid propagation and typically complete fracture of the structure. Fatigue has traditionally been associated with the failure of metal components which led to the term metal fatigue. In the nineteenth century, the sudden failing of metal railway axles was thought to be caused by the metal ''crystallising'' because of the brittle appearance of the fracture surface, but this has since been disproved. Most materials, such as composites, plastics and ceramics, seem to experience some sort of fatigue-related failure. To aid in predicting ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion. In the most common use of the word, this means electrochemical oxidation of metal in reaction with an oxidant such as oxygen, hydrogen or hydroxide. Rusting, the formation of iron oxides, is a well-known example of electrochemical corrosion. This type of damage typically produces oxide(s) or salt(s) of the original metal and results in a distinctive orange colouration. Corrosion can also occur in materials other than metals, such as ceramics or polymers, although in this context, the term "degradation" is more common. Corrosion degrades the useful properties of materials and structures including strength, appearance and permeability to liquids and gases. Many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |