|
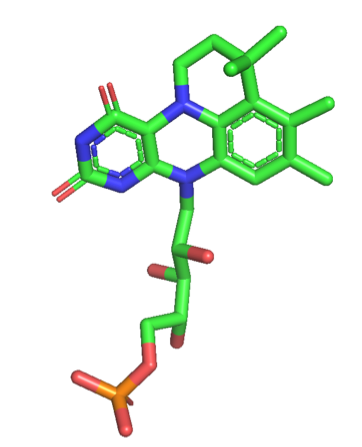
Prenylated Flavin Mononucleotide
Prenylated flavin mononucleotide (prFMN) is a cofactor biosynthesized by the flavin prenyltransferase UbiX and used by UbiD enzymes for reversible decarboxylation reactions. Hence, prFMN is pivotal for catalysis in the ubiquitous microbial UbiD/X system. prFMN is flavin prenylated at the N5 and C6 positions resulting in the formation of a fourth non-aromatic ring. prFMN was discovered in 2015 at the University of Manchester by David Leys' group. : Two studies in 2015 characterized UbiX as a flavin prenyltransferase, supplying prFMN to UbiD/ Fdc1 which utilises the cofactor to catalyse a reversible decarboxylation reaction. Ferulic acid decarboxylase (Fdc1) from ''A. niger'' co-expressed in ''E.coli'' with UbiX from ''E.coli'' (AnFdc1UbiX) once purified had clear spectral differences to singly expressed AnFdc1, and was capable of '' in vitro'' decarboxylation of a range of aromatic carboxylic acids. The atomic resolution of the crystal structure of AnFdc1UbiX, allowed e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound or metallic ion that is required for an enzyme's role as a catalyst (a catalyst is a substance that increases the rate of a chemical reaction). Cofactors can be considered "helper molecules" that assist in biochemical transformations. The rates at which these happen are characterized in an area of study called enzyme kinetics. Cofactors typically differ from ligands in that they often derive their function by remaining bound. Cofactors can be divided into two types: inorganic ions and complex organic molecules called coenzymes. Coenzymes are mostly derived from vitamins and other organic essential nutrients in small amounts. (Note that some scientists limit the use of the term "cofactor" for inorganic substances; both types are included here.) Coenzymes are further divided into two types. The first is called a "prosthetic group", which consists of a coenzyme that is tightly (or even covalently) and permanently bound to a protein. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystal, crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns that repeat along the principal directions of Three-dimensional space (mathematics), three-dimensional space in matter. The smallest group of particles in the material that constitutes this repeating pattern is the unit cell of the structure. The unit cell completely reflects the symmetry and structure of the entire crystal, which is built up by repetitive Translation (geometry), translation of the unit cell along its principal axes. The translation vectors define the nodes of the Bravais lattice. The lengths of the principal axes, or edges, of the unit cell and the angles between them are the lattice constants, also called ''lattice parameters'' or ''cell parameters''. The symmetry properties of the crystal are described by the con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavins
Flavins (from Latin ''flavus'', "yellow") are organic compounds, like their base, pteridine. They are formed by the tricyclic heterocycle isoalloxazine. The biochemical source is the vitamin riboflavin. The flavin moiety is often attached with an adenosine diphosphate to form flavin adenine dinucleotide (FAD), and, in other circumstances, is found as flavin mononucleotide (or FMN), a phosphorylated form of riboflavin. It is in one or the other of these forms that flavin is present as a prosthetic group in flavoproteins. The flavin group is capable of undergoing oxidation-reduction reactions, and can accept either one electron in a two-step process or two electrons at once. Reduction is made with the addition of hydrogen atoms to specific nitrogen atoms on the isoalloxazine ring system: In aqueous solution, flavins are yellow-coloured when oxidized, taking a red colour in the semi-reduced anionic state or blue in the neutral (semiquinone) state, and colourless when totally redu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cofactors
Cofactor may also refer to: * Cofactor (biochemistry), a substance that needs to be present in addition to an enzyme for a certain reaction to be catalysed * A domain parameter in elliptic curve cryptography, defined as the ratio between the order of a group and that of the subgroup * Cofactor (linear algebra), the signed minor of a matrix * Minor (linear algebra), an alternative name for the determinant of a smaller matrix than that which it describes * Shannon cofactor Boole's expansion theorem, often referred to as the Shannon expansion or decomposition, is the identity: F = x \cdot F_x + x' \cdot F_, where F is any Boolean function, x is a variable, x' is the complement of x, and F_xand F_ are F with the argume ..., a term in Boole's (or Shannon's) expansion of a Boolean function See also * Factor (other) {{Disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tautomer
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert. The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydrogen atom within the compound. The phenomenon of tautomerization is called tautomerism, also called desmotropism. Tautomerism is for example relevant to the behavior of amino acids and nucleic acids, two of the fundamental building blocks of life. Care should be taken not to confuse tautomers with depictions of "contributing structures" in chemical resonance. Tautomers are distinct chemical species that can be distinguished by their differing atomic connectivities, molecular geometries, and physicochemical and spectroscopic properties, whereas resonance forms are merely alternative Lewis structure (valence bond theory) depictions of a single chemical species, whose true structure is best described as the "average" of the idealized, hypothe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnamic Acid
Cinnamic acid is an organic compound with the formula C6H5-CH=CH- COOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxylic acid, it occurs naturally in a number of plants. It exists as both a ''cis'' and a ''trans'' isomer, although the latter is more common. Occurrence and production Biosynthesis Cinnamic acid is a central intermediate in the biosynthesis of a myriad of natural products including lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. Its biosynthesis involves the action of the enzyme phenylalanine ammonia-lyase (PAL) on phenylalanine. Natural occurrence It is obtained from oil of cinnamon, or from balsams such as storax. It is also found in shea butter. Cinnamic acid has a honey-like odor; it and its more volatile ethyl ester (ethyl cinnamate) are flavor components ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylpyruvic Acid
Phenylpyruvic acid is the organic compound with the formula C6H5CH2C(O)CO2H. It is a keto acid. Occurrence and properties The compound exists in equilibrium with its E- and Z-enol tautomers. It is a product from the oxidative deamination of phenylalanine. When the activity of the enzyme phenylalanine hydroxylase is reduced, the amino acid phenylalanine accumulates and gets converted into phenylpyruvic acid (phenylpyruvate), which leads to Phenylketonuria (PKU). Preparation and reactions It can be prepared by many methods. Classically it is produced from aminocinnamic acid derivatives. It has been prepared by condensation of benzaldehyde and glycine derivatives to give phenylazlactone, which is then hydrolyzed with acid- or base-catalysis. It can also be synthesized from benzyl chloride by double carbonylation. Reductive amination of phenylpyruvic acid gives phenylalanine. See also * Phenylpyruvate decarboxylase * Phenylpyruvate tautomerase * Phenylketonuria Phenylket ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers. Isomers do not necessarily share similar chemical or physical properties. Two main forms of isomerism are structural or constitutional isomerism, in which ''bonds'' between the atoms differ; and stereoisomerism or spatial isomerism, in which the bonds are the same but the ''relative positions'' of the atoms differ. Isomeric relationships form a hierarchy. Two chemicals might be the same constitutional isomer, but upon deeper analysis be stereoisomers of each other. Two molecules that are the same stereoisomer as each other might be in different conformational forms or be different isotopologues. The depth of analysis depends on the field of study or the chemical and physical properties of interest. The English word "isomer" () is a back-for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PrFMN
Prenylated flavin mononucleotide (prFMN) is a cofactor biosynthesized by the flavin prenyltransferase UbiX and used by UbiD enzymes for reversible decarboxylation reactions. Hence, prFMN is pivotal for catalysis in the ubiquitous microbial UbiD/X system. prFMN is flavin prenylated at the N5 and C6 positions resulting in the formation of a fourth non-aromatic ring. prFMN was discovered in 2015 at the University of Manchester by David Leys' group. : Two studies in 2015 characterized UbiX as a flavin prenyltransferase, supplying prFMN to UbiD/ Fdc1 which utilises the cofactor to catalyse a reversible decarboxylation reaction. Ferulic acid decarboxylase (Fdc1) from ''A. niger'' co-expressed in ''E.coli'' with UbiX from ''E.coli'' (AnFdc1UbiX) once purified had clear spectral differences to singly expressed AnFdc1, and was capable of '' in vitro'' decarboxylation of a range of aromatic carboxylic acids. The atomic resolution of the crystal structure of AnFdc1UbiX, allowed e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prenylation
Prenylation (also known as isoprenylation or lipidation) is the addition of hydrophobic molecules to a protein or a biomolecule. It is usually assumed that prenyl groups (3-methylbut-2-en-1-yl) facilitate attachment to cell membranes, similar to lipid anchors like the GPI anchor, though direct evidence of this has not been observed. Prenyl groups (also called isoprenyl groups, having one hydrogen atom more than isoprene) have been shown to be important for protein–protein binding through specialized prenyl-binding domains. Protein prenylation Protein prenylation involves the transfer of either a farnesyl or a geranylgeranyl moiety to C-terminal cysteine(s) of the target protein. There are three enzymes that carry out prenylation in the cell, farnesyl transferase, Caax protease and geranylgeranyl transferase I. Farnesylation is a type of prenylation, a post-translational modification of proteins by which an isoprenyl group is added to a cysteine residue. It is an important pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic Acid
Aromatic acids are a type of aromatic compound. Included in that class are substances containing an aromatic ring and an organic acid functional group. There are several categories of aromatic acids including: *Phenolic acids: substances containing an aromatic ring and an organic carboxylic acid function (C6-C1 skeleton). *Aromatic amino acids An aromatic amino acid is an amino acid that includes an aromatic ring. Among the 20 standard amino acids, the following are classically considered aromatic: phenylalanine, tryptophan and tyrosine. Although histidine contains an aromatic ring, ... References {{organic-chemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavin Group
Flavins (from Latin ''flavus'', "yellow") are organic compounds, like their base, pteridine. They are formed by the tricyclic heterocycle isoalloxazine. The biochemical source is the vitamin riboflavin. The flavin moiety is often attached with an adenosine diphosphate to form flavin adenine dinucleotide (FAD), and, in other circumstances, is found as flavin mononucleotide (or FMN), a phosphorylated form of riboflavin. It is in one or the other of these forms that flavin is present as a prosthetic group in flavoproteins. The flavin group is capable of undergoing oxidation-reduction reactions, and can accept either one electron in a two-step process or two electrons at once. Reduction is made with the addition of hydrogen atoms to specific nitrogen atoms on the isoalloxazine ring system: In aqueous solution, flavins are yellow-coloured when oxidized, taking a red colour in the semi-reduced anionic state or blue in the neutral (semiquinone) state, and colourless when totally red ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |