PrFMN on:
[Wikipedia]
[Google]
[Amazon]
Prenylated flavin mononucleotide (prFMN) is a cofactor biosynthesized by the flavin prenyltransferase UbiX and used by UbiD enzymes for reversible decarboxylation reactions. Hence, prFMN is pivotal for catalysis in the ubiquitous microbial UbiD/X system.
prFMN is flavin prenylated at the N5 and C6 positions resulting in the formation of a fourth non-aromatic ring.
prFMN was discovered in 2015 at the University of Manchester by David Leys' group.
: Two studies in 2015 characterized UbiX as a flavin prenyltransferase, supplying prFMN to UbiD/ Fdc1 which utilises the cofactor to catalyse a reversible
Two studies in 2015 characterized UbiX as a flavin prenyltransferase, supplying prFMN to UbiD/ Fdc1 which utilises the cofactor to catalyse a reversible 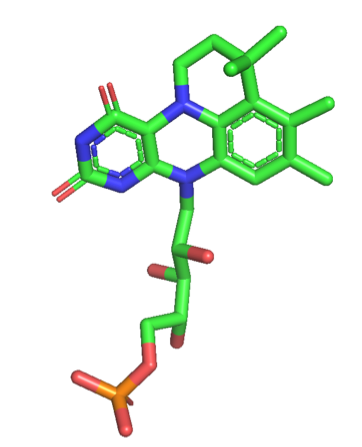
 Two studies in 2015 characterized UbiX as a flavin prenyltransferase, supplying prFMN to UbiD/ Fdc1 which utilises the cofactor to catalyse a reversible
Two studies in 2015 characterized UbiX as a flavin prenyltransferase, supplying prFMN to UbiD/ Fdc1 which utilises the cofactor to catalyse a reversible decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is t ...
reaction. Ferulic acid decarboxylase
Ferulic acid decarboxylases (Fdc) are decarboxylase enzymes capable of the reversible decarboxylation of aromatic carboxylic acids such as ferulic acid and cinnamic acid. Fdc's are fungal homologues of the '' E.coli'' UbiD enzyme which is i ...
(Fdc1) from ''A. niger'' co-expressed in ''E.coli'' with UbiX from ''E.coli'' (AnFdc1UbiX) once purified had clear spectral differences to singly expressed AnFdc1, and was capable of '' in vitro'' decarboxylation of a range of aromatic carboxylic acids. The atomic resolution of the crystal structure of AnFdc1UbiX, allowed elucidation of the structure of the modified FMN cofactor classified as prFMN. The crystal structure revealed an isopentenyl-adduct to the N5-C6 of FMN, with the modifications branched nature and the position of the covalent linkages with flavin suggesting prenylation.
:
PrFMNOx
UbiD activation by UbiX/prFMN was found to be dependent on oxygen suggesting that the reduced prFMN product of UbiX is oxidised to the catalytically relevant form. Several variations of the oxidised prFMN (prFMNox) cofactor were observed: prFMNiminium, hydroxylated prFMNiminium and prFMNketimine. Determination of the prFMNisomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Iso ...
that was catalytically relevant involved incubation of AnFdc1UbiX with phenylpyruvate (of which a small proportion is α-hydroxycinnamic acid which closely resembles cinnamic acid - a model substrate). Incubation with phenylpyruvate lead to an altered UV-Vis spectrum and reversible enzyme inhibition. The crystal structure of AnFdc1UbiX with phenylpyruvate revealed a bond between C1’ of prFMNiminium and a phenylacetaldehyde adduct – a species that can be formed by decarboxylation of α-hydroxycinnamic acid and tautomerization of the α-hydroxystyrene prFMNiminium adduct.
This observation confirmed that it is the prFMNiminium that is the catalytically relevant cofactor.
References
{{reflist Cofactors Flavins Heterocyclic compounds with 4 rings Organophosphates