|
Potential Determining Ion
When placed into solution, salts begin to dissolve and form ions. This is not always in equal proportion, due to the preference of an ion to be dissolved in a given solution. The ability of an ion to preferentially dissolve (as a result of unequal activities) over its counterion is classified as the potential determining ion. The properties of this ion are strongly related to the surface potential present on a corresponding solid. This unequal property between corresponding ions results in a net surface charge. In some cases this arises because one of the ions freely leaves a corresponding solid and the other does not or it is bound to the solid by some other means. Adsorption of an ion to the solid may result in the solid acting as an electrode. (e.g., H+ and OH− on the surfaces of clays). In a colloidal dispersed system, ion dissolution arises, where the dispersed particles exist in equilibrium with their saturated counterpart, for example: :NaCl(s) Na+(aq) + Cl−(aq) The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Colloid
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend the definition to include substances like aerosols and gels. The term colloidal suspension refers unambiguously to the overall mixture (although a narrower sense of the word '' suspension'' is distinguished from colloids by larger particle size). A colloid has a dispersed phase (the suspended particles) and a continuous phase (the medium of suspension). The dispersed phase particles have a diameter of approximately 1 nanometre to 1 micrometre. Some colloids are translucent because of the Tyndall effect, which is the scattering of light by particles in the colloid. Other colloids may be opaque or have a slight color. Colloidal suspensions are the subject of interface and colloid science. This field of study was introduced in 1845 by Ital ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
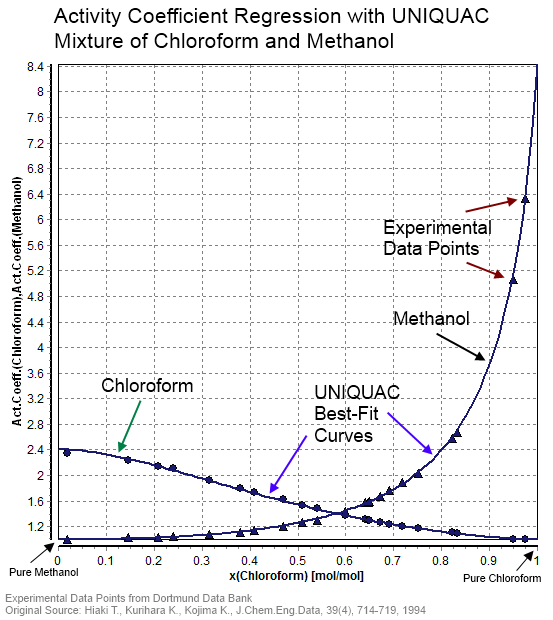
Activity Coefficient
In thermodynamics, an activity coefficient is a factor used to account for deviation of a mixture of chemical substances from ideal behaviour. In an ideal mixture, the microscopic interactions between each pair of chemical species are the same (or macroscopically equivalent, the enthalpy change of solution and volume variation in mixing is zero) and, as a result, properties of the mixtures can be expressed directly in terms of simple concentrations or partial pressures of the substances present e.g. Raoult's law. Deviations from ideality are accommodated by modifying the concentration by an ''activity coefficient''. Analogously, expressions involving gases can be adjusted for non-ideality by scaling partial pressures by a fugacity coefficient. The concept of activity coefficient is closely linked to that of activity in chemistry. Thermodynamic definition The chemical potential, \mu_\mathrm, of a substance B in an ideal mixture of liquids or an ideal solution is given by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solubility Product
Solubility equilibrium is a type of dynamic equilibrium that exists when a chemical compound in the solid state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation, or with chemical reaction with another constituent of the solution, such as acid or alkali. Each solubility equilibrium is characterized by a temperature-dependent ''solubility product'' which functions like an equilibrium constant. Solubility equilibria are important in pharmaceutical, environmental and many other scenarios. Definitions A solubility equilibrium exists when a chemical compound in the solid state is in chemical equilibrium with a solution containing the compound. This type of equilibrium is an example of dynamic equilibrium in that some individual molecules migrate between the solid and solution phases such that the rates of dissolution and precipitation are equal to one another. When equilibrium is established and the solid has not all dissolv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Ion
A hydrogen ion is created when a hydrogen atom loses or gains an electron. A positively charged hydrogen ion (or proton) can readily combine with other particles and therefore is only seen isolated when it is in a gaseous state or a nearly particle-free space. Due to its extremely high charge density of approximately 2×1010 times that of a sodium ion, the bare hydrogen ion cannot exist freely in solution as it readily hydrates, i.e., bonds quickly. The hydrogen ion is recommended by IUPAC as a general term for all ions of hydrogen and its isotopes. Depending on the charge of the ion, two different classes can be distinguished: positively charged ions and negatively charged ions. Cation (positively charged) A hydrogen atom is made up of a nucleus with charge +1, and a single electron. Therefore, the only positively charged ion possible has charge +1. It is noted H+. Depending on the isotope in question, the hydrogen cation has different names: * Hydron: general name refe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Point Of Zero Charge
The point of zero charge (pzc) is generally described as the pH at which the net charge of total particle surface (i.e. absorbent's surface) is equal to zero, which concept has been introduced in the studies dealt with colloidal flocculation to explain pH affecting the phenomenon. A related concept in electrochemistry is the electrode potential at the point of zero charge. Generally, the pzc in electrochemistry is the value of the negative decimal logarithm of the activity of the potential-determining ion in the bulk fluid. IUPAC Gold Book The pzc is of fundamental importance in surface science. For example, in the field of environmental science, it determines how easily a substrate is able to adsorb potentially harmful ions. It also has countless applications in technology of colloids, e.g., flotation of minerals. Therefore, the pzc value has been examined in many application of adsorption to the environmental science. The pzc value is typically obtained by titrations and se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |