|
Potassium Tetrafluoronickelate
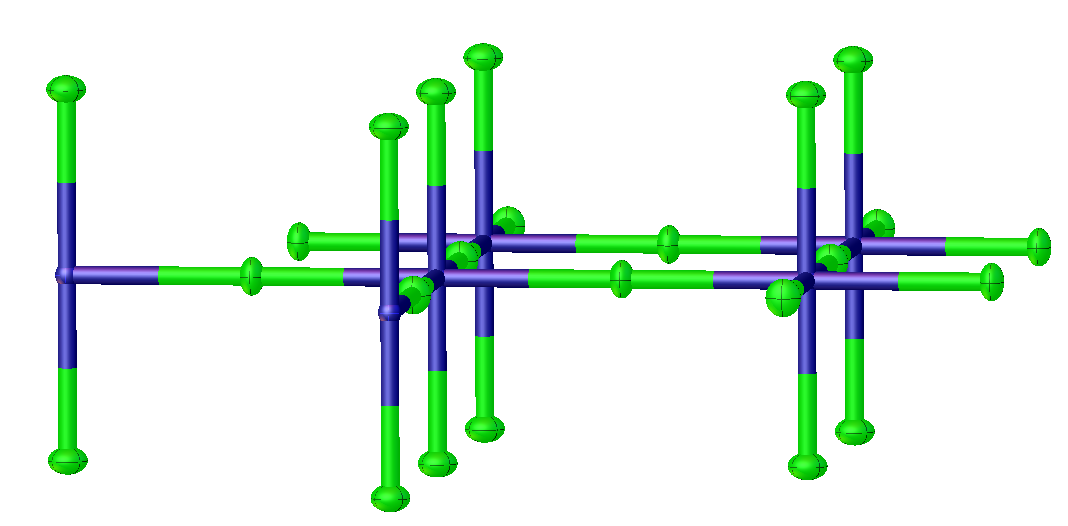
Potassium tetrafluoronickelate is the inorganic compound with the formula K2NiF4. It features octahedral (high spin) Ni centers with Ni-F bond lengths of 2.006 Å. This green solid is a salt of tetrafluoronickelate. It is prepared by melting a mixture of nickel(II) fluoride, potassium fluoride, and potassium bifluoride. The compound adopts a perovskite-like structure consisting of layers of octahedral Ni centers interconnected by doubly bridging fluoride ligands. The layers are interconnected by potassium cations. It is one of the principal Ruddlesden-Popper phases. Early discoveries on cuprate superconductors focused on compounds with structures closely related to K2NiF4, e.g. lanthanum cuprate and derivative lanthanum barium copper oxide Lanthanum barium copper oxide, or LBCO, is an inorganic compound with the formula CuBa0.15La1.85O4. It is a black solid produced by heating an intimate mixture of barium oxide, copper(II) oxide, and lanthanum oxide in the presence of oxyg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting point of modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrafluoronickelate
The fluoronickelates are a class of chemical compounds containing an anion with nickel at its core, surrounded by fluoride ions which act as ligands. This makes it a fluoroanion In chemistry, a fluoroanion or fluorometallate anion is a polyatomic anion that contains one or more fluorine atoms. The ions and salts form from them are also known as complex fluorides. They can occur in salts, or in solution, but seldom as pu .... The nickel atom can be in a range of oxidation states from +2, +3 to +4. The hexafluoronickelate(IV)2− ion NiF62− contains nickel in the maximal +4 state, and is in octahedral coordination by the fluoride atoms. It forms a commercially available salt Potassium hexafluoronickelate(IV) K2NiF6. Solid double salts can also contain tetrafluoronickelate NiF4 eg K2NiF4. References {{fluorine compounds Nickel complexes Fluoro complexes Fluorometallates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel(II) Fluoride
Nickel(II) fluoride is the chemical compound with the formula NiF2. It is an ionic compound of nickel and fluorine and forms yellowish to green tetragonal crystals. Unlike many fluorides, NiF2 is stable in air. Nickel(II) fluoride is also produced when nickel metal is exposed to fluorine. In fact, NiF2 comprises the passivating surface that forms on nickel alloys (e.g. monel) in the presence of hydrogen fluoride or elemental fluorine. For this reason, nickel and its alloys are suitable materials for storage and transport these fluorine and related fluorinating agents. NiF2 is also used as a catalyst for the synthesis of chlorine pentafluoride. Preparation and structure NiF2 is prepared by treatment of anhydrous nickel(II) chloride with fluorine at 350 °C: :NiCl2 + F2 → NiF2 + Cl2 The corresponding reaction of cobalt(II) chloride results in oxidation of the cobalt, whereas nickel remains in the +2 oxidation state after fluorination because its +3 oxidation state i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Fluoride
Potassium fluoride is the chemical compound with the formula KF. After hydrogen fluoride, KF is the primary source of the fluoride ion for applications in manufacturing and in chemistry. It is an alkali halide and occurs naturally as the rare mineral carobbiite. Solutions of KF will etch glass due to the formation of soluble fluorosilicates, although HF is more effective. Preparation Potassium fluoride is prepared by dissolving potassium carbonate in hydrofluoric acid. Evaporation of the solution forms crystals of potassium bifluoride. The bifluoride on heating yields potassium fluoride: : K2CO3 + 4HF -> 2KHF2 + CO2 ^ + H2O : KHF2 -> KF + HF ^ Platinum or heat resistant plastic containers are often used for these operations. Potassium chloride converts to KF upon treatment with hydrogen fluoride. In this way, potassium fluoride is recyclable. Crystalline properties KF crystallizes in the cubic NaCl crystal structure. The lattice parameter at room temperature is 0.266 nm. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Bifluoride
Potassium bifluoride is the inorganic compound with the formula . This colourless salt consists of the potassium cation () and the bifluoride anion (). The salt is used as an etchant for glass. Sodium bifluoride is related and is also of commercial use as an etchant as well as in cleaning products. Synthesis and reactions The salt was prepared by Edmond Frémy by treating potassium carbonate or potassium hydroxide with hydrofluoric acid: : With one more equivalent of HF, (CAS#12178-06-2, m.p. 71.7 C) is produced: : Thermal decomposition of gives hydrogen fluoride: : Applications The industrial production of fluorine entails the electrolysis of molten and . The electrolysis of was first used by Henri Moissan in 1886. See also * Ammonium bifluoride Ammonium hydrogen fluoride is the inorganic compound with the formula or . It is produced from ammonia and hydrogen fluoride. This colourless salt is a glass- etchant and an intermediate in a once-contemplated route to hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perovskite
Perovskite (pronunciation: ) is a calcium titanium oxide mineral composed of calcium titanate (chemical formula ). Its name is also applied to the class of compounds which have the same type of crystal structure as (XIIA2+VIB4+X2−3), known as the perovskite structure. Many different cations can be embedded in this structure, allowing the development of diverse engineered materials. History The mineral was discovered in the Ural Mountains of Russia by Gustav Rose in 1839 and is named after Russian mineralogist Lev Perovski (1792–1856). Perovskite's notable crystal structure was first described by Victor Goldschmidt in 1926 in his work on tolerance factors. The crystal structure was later published in 1945 from X-ray diffraction data on barium titanate by Helen Dick Megaw. Occurrence Found in the Earth's mantle, perovskite's occurrence at Khibina Massif is restricted to the silica under-saturated ultramafic rocks and foidolites, due to the instability in a paragenesis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ruddlesden-Popper Phase
Ruddlesden-Popper (RP) phases are a type of perovskite structure that consists of two-dimensional perovskite-like slabs interleaved with cations. The general formula of an RP phase is ''An+1BnX3n+1'', where ''A'' and ''B'' are cations, ''X'' is an anion (e.g., oxygen), and ''n'' is the number of octahedral layers in the perovskite-like stack. Generally, it has a phase structure that results from the intergrowth of perovskite-type and NaCl-type (i.e., rocksalt-type) structures. These phases are named after S.N. Ruddlesden and P. Popper, who first synthesized and described a Ruddlesden-Popper structure in 1957. Crystal structure The general RP formula ''An+1BnX3n+1'' can be written ''An-1A’2BnX3n+1'', where ''A'' and ''A’'' are alkali, alkaline earth, or rare earth metals and ''B'' is a transition metal. The ''A'' cations are located in the perovskite layer and are 12-fold cuboctahedral coordinated by the anions (CN = 12). The ''A’'' cations have a coordination number of 9 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanum Cuprate
Lanthanum cuprate usually refers to the inorganic compound with the formula CuLa2O4. The name implies that the compound consists of a cuprate (CuOn]2n-) salt of lanthanum (La3+). In fact it is a highly covalent solid. It is prepared by high temperature reaction of lanthanum oxide and copper(II) oxide follow by annealing under oxygen. The material adopts a tetragonal structure related to potassium tetrafluoronickelate (K2NiF4), which is orthorhombic. Replacement of some lanthanum by barium gives the quaternary phase CuLa1.85Ba0.15O4, called lanthanum barium copper oxide. That doped material displays superconductivity at −243 K, which at the time of its discovery was a high temperature. This discovery initiated research on cuprate superconductors and was the basis of a Nobel Prize in Physics to Georg Bednorz Johannes Georg Bednorz (; born 16 May 1950) is a German physicist who, together with K. Alex Müller, discovered high-temperature superconductivity in ceramics, for w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanum Barium Copper Oxide
Lanthanum barium copper oxide, or LBCO, is an inorganic compound with the formula CuBa0.15La1.85O4. It is a black solid produced by heating an intimate mixture of barium oxide, copper(II) oxide, and lanthanum oxide in the presence of oxygen. The material was discovered in 1986 and was the first high temperature superconductor. Johannes Georg Bednorz and K. Alex Müller shared the 1987 Nobel Prize in physics for the discovery that this material exhibits superconductivity at the then unusually high temperature. This finding led to intense and fruitful efforts to generate other cuprate superconductor Cuprate superconductors are a family of high-temperature superconducting materials made of layers of copper oxides (CuO2) alternating with layers of other metal oxides, which act as charge reservoirs. At ambient pressure, cuprate superconductor ...s. Lanthanum barium copper oxide is related to the far simpler compound lanthanum cuprate, which has a similar structure. In lan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel Compounds
Nickel compounds are chemical compounds containing the element nickel which is a member of the group 10 of the periodic table. Most compounds in the group have an oxidation state of +2. Nickel is classified as a transition metal with nickel(II) having much chemical behaviour in common with iron(II) and cobalt(II). Many salts of nickel(II) are isomorphous with salts of magnesium due to the ionic radii of the cations being almost the same. Nickel forms many coordination complexes. Nickel tetracarbonyl was the first pure metal carbonyl produced, and is unusual in its volatility. Metalloproteins containing nickel are found in biological systems. Nickel forms simple binary compounds with non metals including halogens, chalcogenides, and pnictides. Nickel ions can act as a cation in salts with many acids, including common oxoacids. Salts of the hexaaqua ion (Ni2+) are especially well known. Many double salts containing nickel with another cation are known. There are organic acid salts. N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorides
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typically have distinctive bitter tastes, and are odorless. Its salts and minerals are important chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Fluoride is classified as a weak base since it only partially associates in solution, but concentrated fluoride is corrosive and can attack the skin. Fluoride is the simplest fluorine anion. In terms of charge and size, the fluoride ion resembles the hydroxide ion. Fluoride ions occur on Earth in several minerals, particularly fluorite, but are present only in trace quantities in bodies of water in nature. Nomenclature Fluorides include compounds that contain ionic fluoride and those in which fluoride does not dissociate. The nomenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_crystallographic_standard_alignment.png)

-chloride-hexahydrate-sample.jpg)