|
Polychlorotrifluoroethylene
Polychlorotrifluoroethylene (PCTFE or PTFCE) is a thermoplastic chlorofluoropolymer with the molecular formula , where ''n'' is the number of monomer units in the polymer molecule. It is similar to polytetrafluoroethene (PTFE), except that it is a homopolymer of the monomer chlorotrifluoroethylene (CTFE) instead of tetrafluoroethene. It has the lowest water vapor transmission rate of any plastic. History It was discovered in 1934 by Fritz Schloffer and Otto Scherer who worked at IG Farben Company, Germany. Trade names After World War II, PCTFE was commercialized under the trade name of Kel-F 81 by M. W. Kellogg Company in early 1950s. The name "Kel-F" was derived from "Kellogg" and "fluoropolymer", which also represents other fluoropolymers like the copolymer poly(chlorotrifluoroethylene-co-vinylidene fluoride) (Kel-F 800). These were acquired by 3M Company in 1957. But 3M discontinued manufacturing of Kel-F by 1996. PCTFE resin is now manufactured in different trade names ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoropolymer
A fluoropolymer is a fluorocarbon-based polymer with multiple carbon–fluorine bonds. It is characterized by a high resistance to solvents, acids, and bases. The best known fluoropolymer is polytetrafluoroethylene under the brand name "Teflon," trademarked by the DuPont Company. History In 1938, polytetrafluoroethylene (DuPont brand name Teflon) was discovered by accident by a recently hired DuPont Ph.D., Roy J. Plunkett. While working with tetrafluoroethylene gas to develop refrigerants, he noticed that a previously pressurized cylinder had no pressure remaining. In dissecting the cylinder, he found a mass of white solid in a quantity similar to that of the tetrafluoroethylene gas. It was determined that this material was a new-to-the-world polymer. Tests showed the substance was resistant to corrosion from most acids, bases and solvents and had better high temperature stability than any other plastic. By early 1941, a crash program was making substantial quantities of P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Daikin
is a Japanese multinational air conditioning manufacturing company headquartered in Osaka. It has operations in Japan, China, Australia, the United States, India, Southeast Asia, Europe, the Middle East, Latin America, and Africa. Daikin is the inventor of variable refrigerant flow (VRF) air conditioners. Having failed to trademark "VRF", Daikin later remarketed the systems as "VRV" (variable refrigerant volume). Daikin is an innovator in the split system air conditioning market, having made the first split and multi-split air conditioners. Daikin co-developed the R-410A refrigerant with Carrier. History Daikin Industries Ltd was founded in 1924 as Osaka Kinzoku Kogyosho LP by Akira Yamada. In 1953, Daiflon or polychlorotrifluoroethylene was developed. In 1963 the company was renamed Daikin Kogyo Co Ltd and developed Neoflon. In 1982 it was renamed to the current Daikin Industries Ltd. Daikin entered the North American air conditioning market in 2004. In 2006, Daikin Indust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorotrifluoroethylene
Chlorotrifluoroethylene (CTFE) is a chlorofluorocarbon with chemical formula CFCl=CF2. It is commonly used as a refrigerant in cryogenic applications. CTFE has a carbon-carbon double bond and so can be polymerized to form polychlorotrifluoroethylene or copolymerized to produce the plastic ECTFE. PCTFE has the trade name Neoflon PCTFE from Daikin Industries in Japan, and it used to be produced under the trade name Kel-F from 3M Corporation in Minnesota. Production and reactions Chlorotrifluoroethylene is produced commercially by the dechlorination of 1,1,2-trichloro-1,2,2-trifluoroethane with zinc: :CFCl2-CF2Cl + Zn → CClF=CF2 + ZnCl2 In 2012, an estimated 1–10 million pounds were produced commercially in the United States. The thermal dimerization of chlorotrifluoroethylene gives 1,2-dichloro-1,2,3,3,4,4-hexafluorocyclobutane. Dichlorination of the latter gives hexafluorocyclobutene Hexafluorocyclobutene is the organofluorine compound with the formula (CF2)2(CF)2. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emulsion Polymerization
Emulsion polymerization is a type of radical polymerization that usually starts with an emulsion incorporating water, monomer, and surfactant. The most common type of emulsion polymerization is an oil-in-water emulsion, in which droplets of monomer (the oil) are emulsified (with surfactants) in a continuous phase of water. Water-soluble polymers, such as certain polyvinyl alcohols or hydroxyethyl celluloses, can also be used to act as emulsifiers/stabilizers. The name "emulsion polymerization" is a misnomer that arises from a historical misconception. Rather than occurring in emulsion droplets, polymerization takes place in the latex/colloid particles that form spontaneously in the first few minutes of the process. These latex particles are typically 100 nm in size, and are made of many individual polymer chains. The particles are prevented from coagulating with each other because each particle is surrounded by the surfactant ('soap'); the charge on the surfactant repels other pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free-radical Polymerization
In polymer chemistry, free-radical polymerization (FRP) is a method of polymerization by which a polymer forms by the successive addition of free-radical building blocks (repeat units). Free radicals can be formed by a number of different mechanisms, usually involving separate initiator molecules. Following its generation, the initiating free radical adds (nonradical) monomer units, thereby growing the polymer chain. Free-radical polymerization is a key synthesis route for obtaining a wide variety of different polymers and materials composites. The relatively non-specific nature of free-radical chemical interactions makes this one of the most versatile forms of polymerization available and allows facile reactions of polymeric free-radical chain ends and other chemicals or substrates. In 2001, 40 billion of the 110 billion pounds of polymers produced in the United States were produced by free-radical polymerization. Free-radical polymerization is a type of chain-growth polymeriza ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solution Polymerization
Solution polymerization is a method of industrial polymerization. In this procedure, a monomer is dissolved in a non-reactive solvent that contains a catalyst or initiator. The reaction results in a polymer which is also soluble in the chosen solvent. Heat released by the reaction is absorbed by the solvent, reducing the reaction rate. Moreover, the viscosity of the reaction mixture is reduced, preventing autoacceleration at high monomer concentrations. A decrease in viscosity of the reaction mixture by dilution also aids heat transfer, one of the major issues connected with polymer production, since most polymerizations are exothermic reactions. Once the desired conversion is reached, excess solvent must be removed to obtain the pure polymer. Accordingly, solution polymerization is primarily used in applications where the presence of a solvent is desired anyway, as is the case for varnish and adhesives. Another application of polymer solutions includes the manufacture of fibers b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bulk Polymerization
Bulk polymerization or mass polymerization is carried out by adding a soluble radical initiator to pure monomer in liquid state. The initiator should dissolve in the monomer. The reaction is initiated by heating or exposing to radiation. As the reaction proceeds the mixture becomes more viscous. The reaction is exothermic and a wide range of molecular masses are produced. Bulk polymerization is carried out in the absence of any solvent or dispersant and is thus the simplest in terms of formulation. It is used for most step-growth polymers and many types of chain-growth polymers. In the case of chain-growth reactions, which are generally exothermic, the heat evolved may cause the reaction to become too vigorous and difficult to control unless efficient cooling is used. Advantages and disadvantages Bulk polymerization has several advantages over other methods, these advantages are:Abdullah Youssef, Abdal-Rhman. (2019). Solution & Bulk polymerization. 10.13140/RG.2.2.16472.96001/2. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Suspension Polymerization
Suspension polymerization is a heterogeneous radical polymerization process that uses mechanical agitation to mix a monomer or mixture of monomers in a liquid phase, such as water, while the monomers polymerize, forming spheres of polymer. The monomer droplets (size of the order 10-1000 μm) are suspended in the liquid phase. The individual monomer droplets can be considered as undergoing bulk polymerization. The liquid phase outside these droplets help in better conduction of heat and thus tempering the increase in temperature. While choosing a liquid phase for suspension polymerization, low viscosity, high thermal conductivity and low temperature variation of viscosity are generally preferred. The primary advantage of suspension polymerization over other types of polymerization is that a higher degree of polymerization can be achieved without monomer boil-off. During this process, there is often a possibility of these monomer droplets to stick to each other and cause creaming i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermoplastic
A thermoplastic, or thermosoft plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling. Most thermoplastics have a high molecular weight. The polymer chains associate by intermolecular forces, which weaken rapidly with increased temperature, yielding a viscous liquid. In this state, thermoplastics may be reshaped and are typically used to produce parts by various polymer processing techniques such as injection molding, compression molding, calendering, and extrusion. Thermoplastics differ from thermosetting polymers (or "thermosets"), which form irreversible chemical bonds during the curing process. Thermosets do not melt when heated, but typically decompose and do not reform upon cooling. Above its glass transition temperature and below its melting point, the physical properties of a thermoplastic change drastically without an associated phase change. Some thermoplastics do not fully crystallize ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
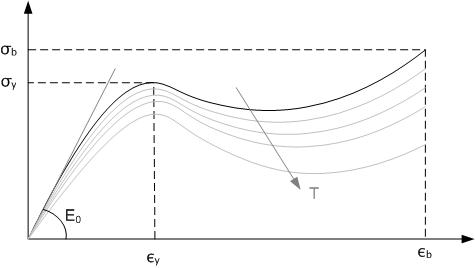
Tensile Strength
Ultimate tensile strength (UTS), often shortened to tensile strength (TS), ultimate strength, or F_\text within equations, is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials the ultimate tensile strength is close to the yield point, whereas in ductile materials the ultimate tensile strength can be higher. The ultimate tensile strength is usually found by performing a tensile test and recording the engineering stress versus strain. The highest point of the stress–strain curve is the ultimate tensile strength and has units of stress. The equivalent point for the case of compression, instead of tension, is called the compressive strength. Tensile strengths are rarely of any consequence in the design of ductile members, but they are important with brittle members. They are tabulated for common materials such as alloys, composite materials, ceramics, plastics, and wood. Definition The ultimate tensile streng ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allied Chemical Corporation
Allied Corp. was a major American company with operations in the chemical, aerospace, automotive, oil and gas industries. It was initially formed in 1920 as the Allied Chemical and Dye Corporation as an amalgamation of five chemical companies. In 1958, it was renamed Allied Chemical Corporation when it diversified into oil and gas exploration. Allied Chemical then became Allied Corporation in 1981. In 1985, Allied merged with the Signal Companies to become AlliedSignal. AlliedSignal would eventually acquire Honeywell in 1999 and then adopt its name. History During World War I, Imperial Germany controlled much of the world's chemical production. This resulted in critical shortages of certain dyes, drugs and especially ammonia, a vital compound used to make fertilizers and explosives. Allied Chemical and Dye Corporation In 1920, publisher Eugene Meyer and noted chemist William Ripley Nichols founded Allied Chemical and Dye Corporation in order to address this shortcoming in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coefficient Of Thermal Expansion
Thermal expansion is the tendency of matter to change its shape A shape or figure is a graphics, graphical representation of an object or its external boundary, outline, or external Surface (mathematics), surface, as opposed to other properties such as color, Surface texture, texture, or material type. A pl ..., area, volume, and density in response to a change in temperature, usually not including phase transitions. Temperature is a monotonic function of the average molecular kinetic energy of a substance. When a substance is heated, molecules begin to vibrate and move more, usually creating more distance between themselves. Substances which contract with increasing temperature are unusual, and only occur within limited temperature ranges (see examples below). The relative expansion (also called strain (mechanics), strain) divided by the change in temperature is called the material's coefficient of linear thermal expansion and generally varies with temperature. As energy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |