|
Polybutadiene
Polybutadiene utadiene rubber BRis a synthetic rubber. Polybutadiene rubber is a polymer formed from the polymerization of the monomer 1,3-butadiene. Polybutadiene has a high resistance to wear and is used especially in the manufacture of tires, which consumes about 70% of the production. Another 25% is used as an additive to improve the toughness (impact resistance) of plastics such as polystyrene and acrylonitrile butadiene styrene (ABS). Polybutadiene rubber accounted for about a quarter of total global consumption of synthetic rubbers in 2012. It is also used to manufacture golf balls, various elastic objects and to coat or encapsulate electronic assemblies, offering high electrical resistivity. The IUPAC refers to polybutadiene as poly (buta-1,3-diene). Buna rubber is a term used to describe an early generation of synthetic polybutadiene rubber produced in Germany by Bayer using sodium as a catalyst. History The Russian chemist Sergei Vasilyevich Lebedev was th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Petrochemical
Petrochemicals (sometimes abbreviated as petchems) are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane. The two most common petrochemical classes are olefins (including ethylene and propylene) and aromatics (including benzene, toluene and xylene isomers). Oil refineries produce olefins and aromatics by fluid catalytic cracking of petroleum fractions. Chemical plants produce olefins by steam cracking of natural gas liquids like ethane and propane. Aromatics are produced by catalytic reforming of naphtha. Olefins and aromatics are the building-blocks for a wide range of materials such as solvents, detergents, and adhesives. Olefins are the basis for polymers and oligomers used in plastics, resins, fibers, elastomers, lubricants, and gels. Global ethylene production was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest conjugated diene. Although butadiene breaks down quickly in the atmosphere, it is nevertheless found in ambient air in urban and suburban areas as a consequence of its constant emission from motor vehicles. The name butadiene can also refer to the isomer, 1,2-butadiene, which is a cumulated diene with structure H2C=C=CH−CH3. This allene has no industrial significance. History In 1863, the French chemist E. Caventou isolated butadiene from the pyrolysis of amyl alcohol. This hydrocarbon was identified as butadiene in 1886, after Henry Edward Armstrong isolated it from among the pyrolysis products of petroleum. In 1910, the Russian chemist Sergei Lebedev polymerized butadiene and obtained a materi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tire Manufacturing
Pneumatic tires are manufactured according to relatively standardized processes and machinery, in around 455 tire factories in the world. With over 1 billion tires manufactured worldwide annually, the tire industry is a major consumer of natural rubber. Tire factories start with bulk raw materials such as synthetic rubber (60% -70% of total rubber in the tire industry), carbon black, and chemicals and produce numerous specialized components that are assembled and cured. The tire is an assembly of numerous components that are built up on a drum and then cured in a press under heat and pressure. Heat facilitates a polymerization reaction that crosslinks rubber monomers to create long elastic molecules. Inner liner The inner liner is a calendered halobutyl rubber sheet compounded with additives that result in low air permeability. The inner liner assures that the tire will hold high-pressure air inside, without an inner tube, minimizing diffusion through the rubber structure. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ziegler–Natta Catalyst
A Ziegler–Natta catalyst, named after Karl Ziegler and Giulio Natta, is a catalyst used in the synthesis of polymers of 1-alkenes ( alpha-olefins). Two broad classes of Ziegler–Natta catalysts are employed, distinguished by their solubility: * Heterogeneous supported catalysts based on titanium compounds are used in polymerization reactions in combination with cocatalysts, organoaluminum compounds such as triethylaluminium, Al(C2H5)3. This class of catalyst dominates the industry. * Homogeneous catalysts usually based on complexes of the group 4 metals titanium, zirconium or hafnium. They are usually used in combination with a different organoaluminum cocatalyst, methylaluminoxane (or methylalumoxane, MAO). These catalysts traditionally contain metallocenes but also feature multidentate oxygen- and nitrogen-based ligands. Ziegler–Natta catalysts are used to polymerize terminal alkenes (ethylene and alkenes with the vinyl double bond): :''n'' CH2=CHR → − H2� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Styrene-butadiene
Styrene-butadiene or styrene-butadiene rubber (SBR) describe families of synthetic rubbers derived from styrene and butadiene (the version developed by Goodyear is called Neolite). These materials have good abrasion resistance and good aging stability when protected by additives. In 2012, more than 5.4 million tonnes of SBR were processed worldwide. About 50% of car tires are made from various types of SBR. The styrene/butadiene ratio influences the properties of the polymer: with high styrene content, the rubbers are harder and less rubbery. SBR is not to be confused with the thermoplastic elastomer, styrene-butadiene block copolymer, although being derived from the same monomers. Types of SBR SBR is derived from two monomers, styrene and butadiene. The mixture of these two monomers is polymerized by two processes: from solution (S-SBR) or as an emulsion (E-SBR). E-SBR is more widely used. Emulsion polymerization E-SBR produced by emulsion polymerization is initiated by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
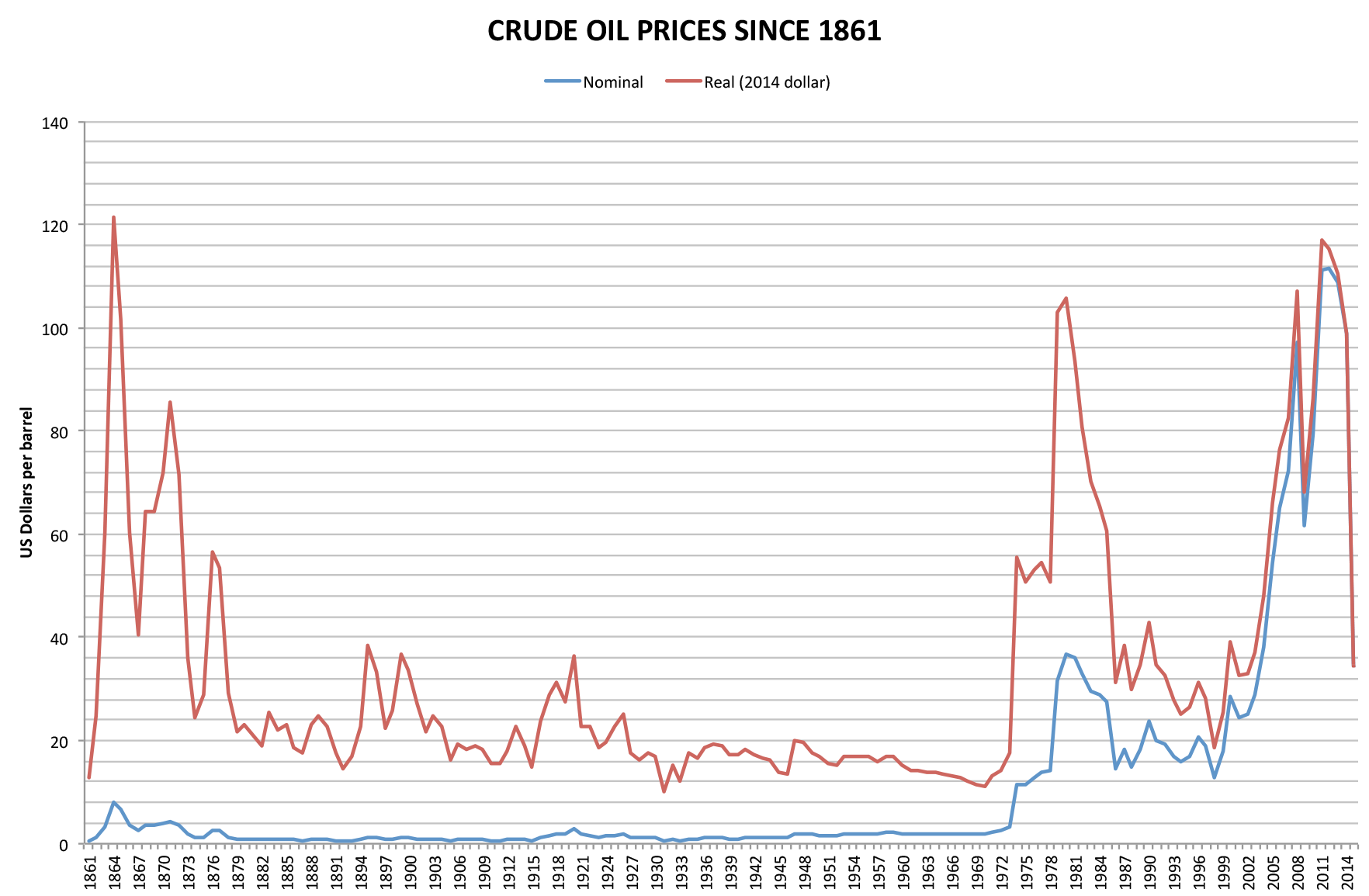
1973 Oil Crisis
The 1973 oil crisis or first oil crisis began in October 1973 when the members of the Organization of Arab Petroleum Exporting Countries (OAPEC), led by Saudi Arabia, proclaimed an oil embargo. The embargo was targeted at nations that had supported Israel during the Yom Kippur War. The initial nations targeted were Canada, Japan, the Netherlands, the United Kingdom and the United States, though the embargo also later extended to Portugal, Rhodesia and South Africa. By the end of the embargo in March 1974, the price of oil had risen nearly 300%, from US to nearly globally; US prices were significantly higher. The embargo caused an oil crisis, or "shock", with many short- and long-term effects on global politics and the global economy. It was later called the "first oil shock", followed by the 1979 oil crisis, termed the "second oil shock". Background Arab-Israeli conflict Ever since the recreation of the State of Israel in 1948 there has been Arab–Israeli confli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Far East
The ''Far East'' was a European term to refer to the geographical regions that includes East and Southeast Asia as well as the Russian Far East to a lesser extent. South Asia is sometimes also included for economic and cultural reasons. The term first came into use in European geopolitical discourse in the 15th century, particularly the British, denoting the Far East as the "farthest" of the three "Easts", beyond the Near East and the Middle East. Likewise, during the Qing dynasty of the 19th and early 20th centuries, the term " Tàixī ()" – i.e., anything further west than the Arab world – was used to refer to the Western countries. Since the mid-20th century, the term has mostly gone out of use for the region in international mass media outlets due to its eurocentric connotations.Reischauer, Edwin and John K Fairbank, ''East Asia: The Great Tradition,'' 1960. The Russian Far East is often excluded due to cultural and ethnic differences, and is often considered as part ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IG Farben
Interessengemeinschaft Farbenindustrie AG (), commonly known as IG Farben (German for 'IG Dyestuffs'), was a German chemical and pharmaceutical conglomerate. Formed in 1925 from a merger of six chemical companies— BASF, Bayer, Hoechst, Agfa, Chemische Fabrik Griesheim-Elektron, and Chemische Fabrik vorm. Weiler Ter Meer—it was seized by the Allies after World War II and divided back into its constituent companies. IG Farben was once the largest company in Europe and the largest chemical and pharmaceutical company in the world. IG Farben scientists made fundamental contributions to all areas of chemistry and the pharmaceutical industry. Otto Bayer discovered the polyaddition for the synthesis of polyurethane in 1937, and three company scientists became Nobel laureates: Carl Bosch and Friedrich Bergius in 1931 "for their contributions to the invention and development of chemical high pressure methods", and Gerhard Domagk in 1939 "for the discovery of the antibacterial ef ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yokohama ADVAN Tires WTCC 2006
is the second-largest city in Japan by population and the most populous municipality of Japan. It is the capital city and the most populous city in Kanagawa Prefecture, with a 2020 population of 3.8 million. It lies on Tokyo Bay, south of Tokyo, in the Kantō region of the main island of Honshu. Yokohama is also the major economic, cultural, and commercial hub of the Greater Tokyo Area along the Keihin Industrial Zone. Yokohama was one of the cities to open for trade with the West following the 1859 end of the policy of seclusion and has since been known as a cosmopolitan port city, after Kobe opened in 1853. Yokohama is the home of many Japan's firsts in the Meiji period, including the first foreign trading port and Chinatown (1859), European-style sport venues (1860s), English-language newspaper (1861), confectionery and beer manufacturing (1865), daily newspaper (1870), gas-powered street lamps (1870s), railway station (1872), and power plant (1882). Yokohama developed ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Styrene
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concentrations have a less pleasant odor. Styrene is the precursor to polystyrene and several copolymers. Approximately 25 million tonnes of styrene were produced in 2010, increasing to around 35 million tonnes by 2018. Natural occurrence Styrene is named after storax balsam (often commercially sold as ''styrax''), the resin of Liquidambar trees of the Altingiaceae plant family. Styrene occurs naturally in small quantities in some plants and foods (cinnamon, coffee beans, balsam trees and peanuts) and is also found in coal tar. History In 1839, the German apothecary Eduard Simon isolated a volatile liquid from the resin (called ''storax'' or ''styrax'' (Latin)) of the American sweetgum tree (''Liquidambar styraciflua''). He called ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Goodrich Corporation
The Goodrich Corporation, formerly the B.F. Goodrich Company, was an American manufacturing company based in Charlotte, North Carolina. Founded in Akron, Ohio in 1870 as Goodrich, Tew & Co. by Benjamin Goodrich, the company name was changed to the "B.F. Goodrich Company" in 1880, to BFGoodrich in the 1980s, and to "Goodrich Corporation" in 2001. Originally a rubber manufacturing company known for automobile tires, the company diversified its manufacturing businesses throughout the twentieth century and sold off its tire business in 1986 to focus on its other businesses, such as aerospace and chemical manufacturing. The BFGoodrich brand name continues to be used by Michelin, who acquired the tire manufacturing business in 1988. Following the acquisition by United Technologies in 2012, Goodrich became a part of UTC Aerospace Systems. In 1869, Benjamin Goodrich purchased the Hudson River Rubber Company, a small business in Hastings-on-Hudson, New York. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |