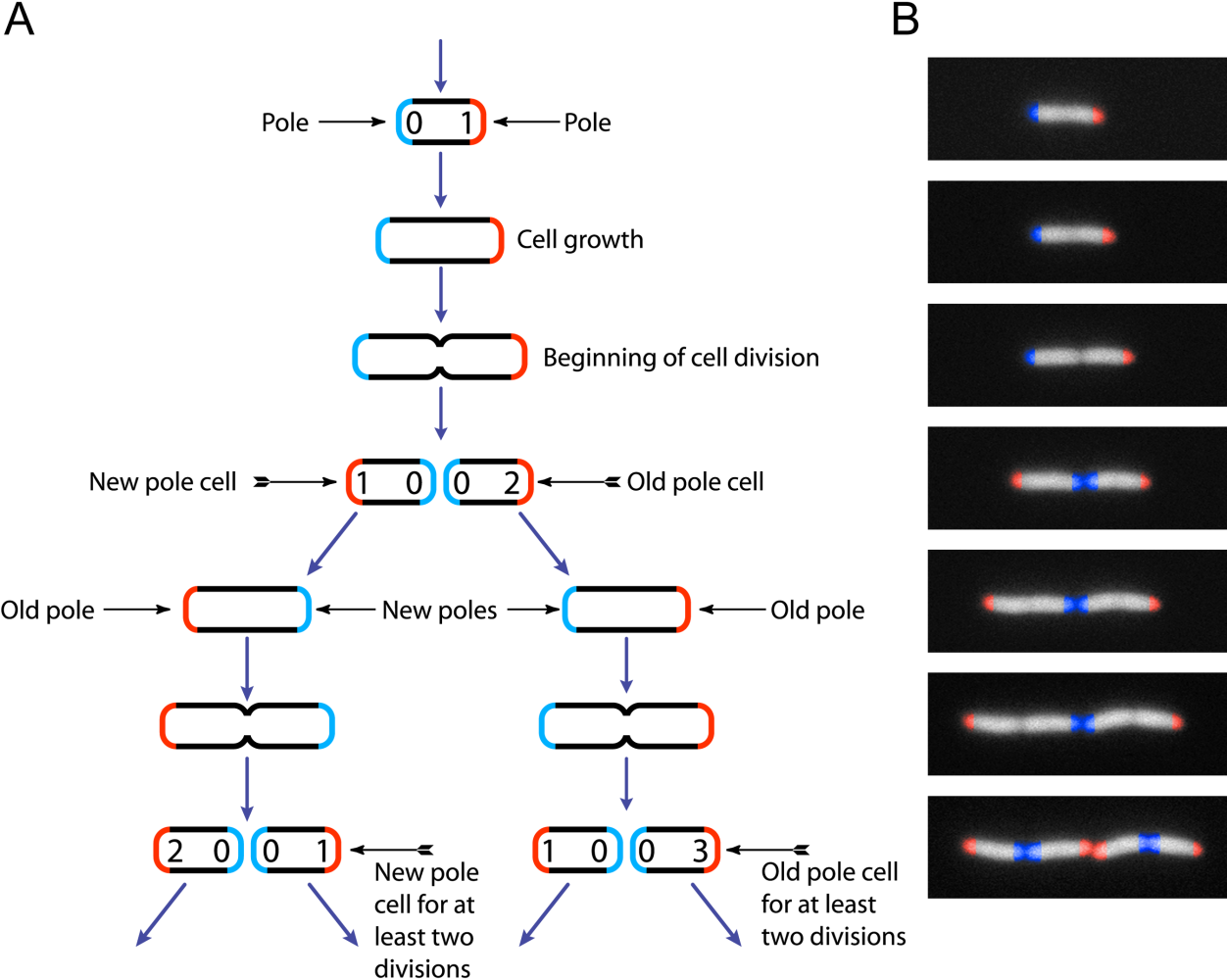
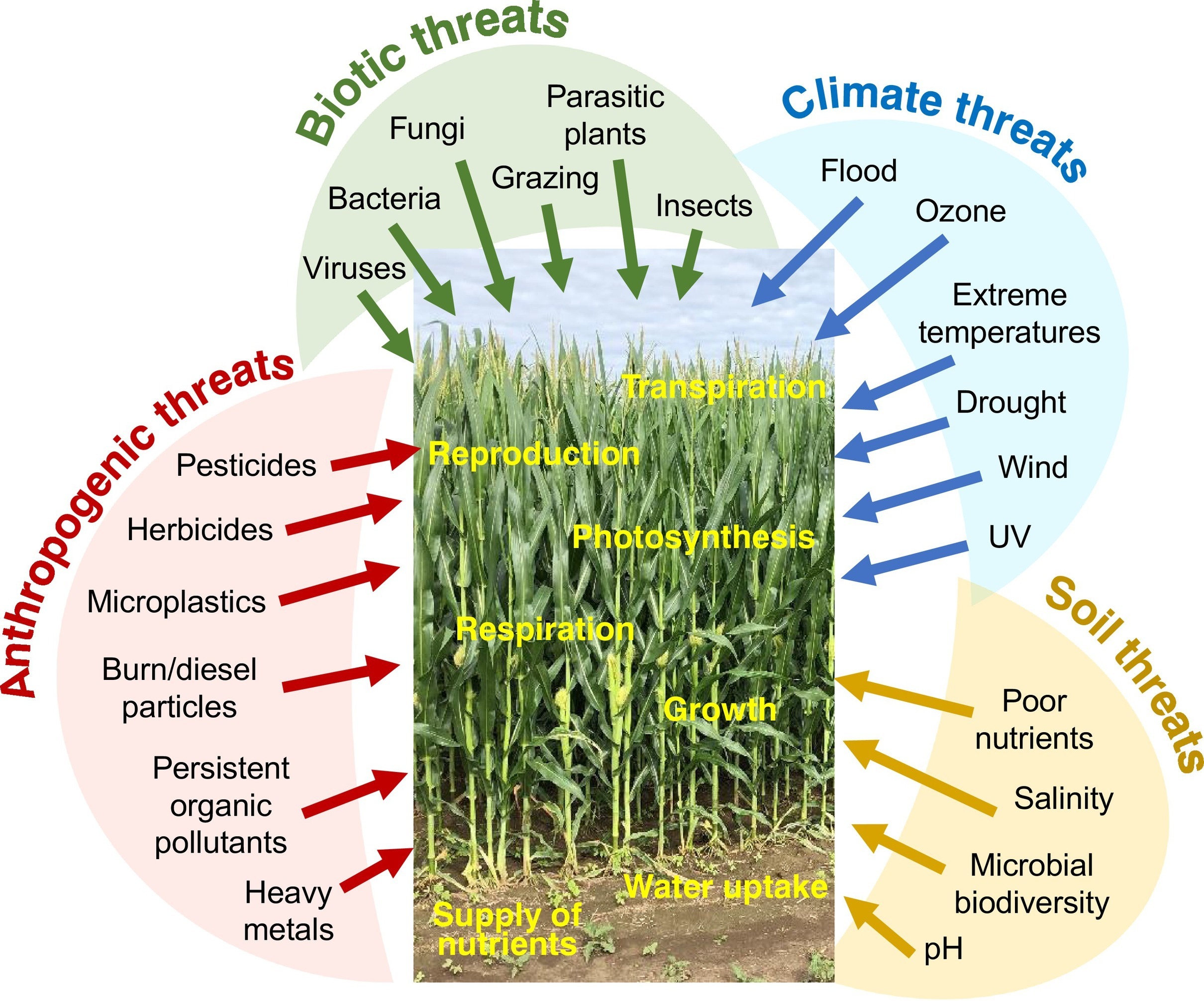
|
Pol V
DNA Polymerase V (Pol V) is a polymerase enzyme involved in DNA repair mechanisms in bacteria, such as ''Escherichia coli''. It is composed of a UmuD' homodimer and a UmuC monomer, forming the UmuD'2C protein complex. It is part of the Y-family of DNA Polymerases, which are capable of performing DNA translesion synthesis (TLS). Translesion polymerases bypass DNA damage lesions during DNA replication - if a lesion is not repaired or bypassed the replication fork can stall and lead to cell death. However, Y polymerases have low sequence fidelity during replication (prone to add wrong nucleotides). When the UmuC and UmuD' proteins were initially discovered in ''E. coli'', they were thought to be agents that inhibit faithful DNA replication and caused DNA synthesis to have high mutation rates after exposure to UV-light. The polymerase function of Pol V was not discovered until the late 1990s when UmuC was successfully extracted, consequent experiments unequivocally proved UmuD'2C is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Escherichia Coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escherichia'' that is commonly found in the lower intestine of warm-blooded organisms. Most ''E. coli'' strains are harmless, but some serotypes ( EPEC, ETEC etc.) can cause serious food poisoning in their hosts, and are occasionally responsible for food contamination incidents that prompt product recalls. Most strains do not cause disease in humans and are part of the normal microbiota of the gut; such strains are harmless or even beneficial to humans (although these strains tend to be less studied than the pathogenic ones). For example, some strains of ''E. coli'' benefit their hosts by producing vitamin K2 or by preventing the colonization of the intestine by pathogenic bacteria. These mutually beneficial relationships between ''E. col ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exonuclease
Exonucleases are enzymes that work by cleaving nucleotides one at a time from the end (exo) of a polynucleotide chain. A hydrolyzing reaction that breaks phosphodiester bonds at either the 3′ or the 5′ end occurs. Its close relative is the endonuclease, which cleaves phosphodiester bonds in the middle (endo) of a polynucleotide chain. Eukaryotes and prokaryotes have three types of exonucleases involved in the normal turnover of mRNA: 5′ to 3′ exonuclease (Xrn1), which is a dependent decapping protein; 3′ to 5′ exonuclease, an independent protein; and poly(A)-specific 3′ to 5′ exonuclease. In both archaea and eukaryotes, one of the main routes of RNA degradation is performed by the multi-protein exosome complex, which consists largely of 3′ to 5′ exoribonucleases. Significance to polymerase RNA polymerase II is known to be in effect during transcriptional termination; it works with a 5' exonuclease (human gene Xrn2) to degrade the newly formed transcript ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
REV1
DNA repair protein REV1 is a protein that in humans is encoded by the ''REV1'' gene. This gene encodes a protein with similarity to the S. cerevisiae mutagenesis protein Rev1. The Rev1 proteins contain a BRCT domain, which is important in protein-protein interactions. A suggested role for the human Rev1-like protein is as a scaffold that recruits DNA polymerases involved in translesion synthesis (TLS) of damaged DNA. Two alternatively spliced transcript variants that encode different proteins have been found. Rev1 is a Y family DNA polymerase; it is sometimes referred to as a ''deoxycytidyl transferase'' because it only inserts deoxycytidine (dC) across from lesions. Whether G, A, T, C, or an abasic site, Rev1 will always add a C. Rev1 has the ability to always add a C, because it uses an arginine as a template which complements well with C. Yet it is believed that Rev1 rarely uses its polymerase activity; rather it is thought that Rev1's primary role is as a protein landing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DNA Synthesis
DNA synthesis is the natural or artificial creation of deoxyribonucleic acid (DNA) molecules. DNA is a macromolecule made up of nucleotide units, which are linked by covalent bonds and hydrogen bonds, in a repeating structure. DNA synthesis occurs when these nucleotide units are joined to form DNA; this can occur artificially (''in vitro'') or naturally (''in vivo''). Nucleotide units are made up of a nitrogenous base (cytosine, guanine, adenine or thymine), pentose sugar (deoxyribose) and phosphate group. Each unit is joined when a covalent bond forms between its phosphate group and the pentose sugar of the next nucleotide, forming a sugar-phosphate backbone. DNA is a complementary, double stranded structure as specific base pairing (adenine and thymine, guanine and cytosine) occurs naturally when hydrogen bonds form between the nucleotide bases. There are several different definitions for DNA synthesis: it can refer to DNA replication - DNA biosynthesis (''in vivo'' DNA amplific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Post-translational Modification
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo PTM to form the mature protein product. PTMs are important components in cell signaling, as for example when prohormones are converted to hormones. Post-translational modifications can occur on the amino acid side chains or at the protein's C- or N- termini. They can extend the chemical repertoire of the 20 standard amino acids by modifying an existing functional group or introducing a new one such as phosphate. Phosphorylation is a highly effective mechanism for regulating the activity of enzymes and is the most common post-translational modification. Many eukaryotic and prokaryotic proteins also have carbohydrate molecules attached to them in a process called glycosyla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteolysis
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, but may also occur by intra-molecular digestion. Proteolysis in organisms serves many purposes; for example, digestive enzymes break down proteins in food to provide amino acids for the organism, while proteolytic processing of a polypeptide chain after its synthesis may be necessary for the production of an active protein. It is also important in the regulation of some physiological and cellular processes including apoptosis, as well as preventing the accumulation of unwanted or misfolded proteins in cells. Consequently, abnormality in the regulation of proteolysis can cause disease. Proteolysis can also be used as an analytical tool for studying proteins in the laboratory, and it may also be used in industry, for example in food proc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Repressor LexA
Repressor LexA or LexA is a transcriptional repressor () that represses SOS response genes coding primarily for error-prone DNA polymerases, DNA repair enzyme Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...s and cell division inhibitors. LexA forms ''de facto'' a two-component regulatory system with RecA, which senses DNA damage at stalled replication forks, forming monofilaments and acquiring an active conformation capable of binding to LexA and causing LexA to cleave itself, in a process called proteolysis, autoproteolysis. DNA damage can be inflicted by the action of antibiotics, bacteriophages, and UV light. Of potential clinical interest is the induction of the SOS response by antibiotics, such as ciprofloxacin. Bacteria require topoisomerases such as DNA gyrase or topoisomer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Negative Regulation
In genetics, an operon is a functioning unit of DNA containing a cluster of genes under the control of a single promoter. The genes are transcribed together into an mRNA strand and either translated together in the cytoplasm, or undergo splicing to create monocistronic mRNAs that are translated separately, i.e. several strands of mRNA that each encode a single gene product. The result of this is that the genes contained in the operon are either expressed together or not at all. Several genes must be ''co-transcribed'' to define an operon. Originally, operons were thought to exist solely in prokaryotes (which includes organelles like plastids that are derived from bacteria), but since the discovery of the first operons in eukaryotes in the early 1990s, more evidence has arisen to suggest they are more common than previously assumed. In general, expression of prokaryotic operons leads to the generation of polycistronic mRNAs, while eukaryotic operons lead to monocistronic mRNAs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RecF Pathway
The RecF pathway, also called the RecFOR pathway, is a pathway of homologous recombination that repairs DNA in bacteria. It repairs breaks that occur on only one of DNA's two strands, known as single-strand gaps. The RecF pathway can also repair double-strand breaks in DNA when the RecBCD pathway, another pathway of homologous recombination in bacteria, is inactivated by mutations. Like the RecBCD pathway, the RecF pathway requires RecA for strand invasion. The two pathways are also similar in their phases of branch migration, in which the Holliday junction slides in one direction, and resolution, in which the Holliday junctions are cleaved apart by enzymes. The RecF pathway begins when RecJ, an exonuclease that cleaves single-stranded DNA in the 5 → 3′ direction, binds to the 5' end of a single-strand gap in DNA and starts moving upstream while cleaving the 5' strand. Although RecJ can function without them, single-strand binding protein (SSBP) and the RecQ helicase g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DnaB Helicase
DnaB helicase is an enzyme in bacteria which opens the replication fork during DNA replication. Although the mechanism by which DnaB both couples ATP hydrolysis to translocation along DNA and denatures the duplex is unknown, a change in the quaternary structure of the protein involving dimerisation of the N-terminal domain has been observed and may occur during the enzymatic cycle. Initially when DnaB binds to dnaA, it is associated with dnaC, a negative regulator. After DnaC dissociates, DnaB binds dnaG. The N-terminal has a multi-helical structure that forms an orthogonal bundle. The C-terminal domain contains an ATP-binding site and is therefore probably the site of ATP hydrolysis. In eukaryotes, helicase function is provided by the MCM (Minichromosome maintenance) complex. The DnaB helicase is the product of the ''dnaB'' gene. The helicase enzyme that is produced is a hexamer in ''E. coli'', as well as in many other bacteria. The energy for DnaB activity is provided by N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stress (biology)
Stress, either physiological, biological or psychological, is an organism's response to a stressor such as an environmental condition. Stress is the body's method of reacting to a condition such as a threat, challenge or physical and psychological barrier. There are two hormones that an individual produces during a stressful situation, these are well known as adrenaline and cortisol. There are two kinds of stress hormone levels. Resting (basal) cortisol levels are normal everyday quantities that are essential for standard functioning. Reactive cortisol levels are increases in cortisol in response to stressors. Stimuli that alter an organism's environment are responded to by multiple systems in the body. In humans and most mammals, the autonomic nervous system and hypothalamic-pituitary-adrenal (HPA) axis are the two major systems that respond to stress. The sympathoadrenal medullary (SAM) axis may activate the fight-or-flight response through the sympathetic nervous system ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |