|
Plastoquinone Reduction
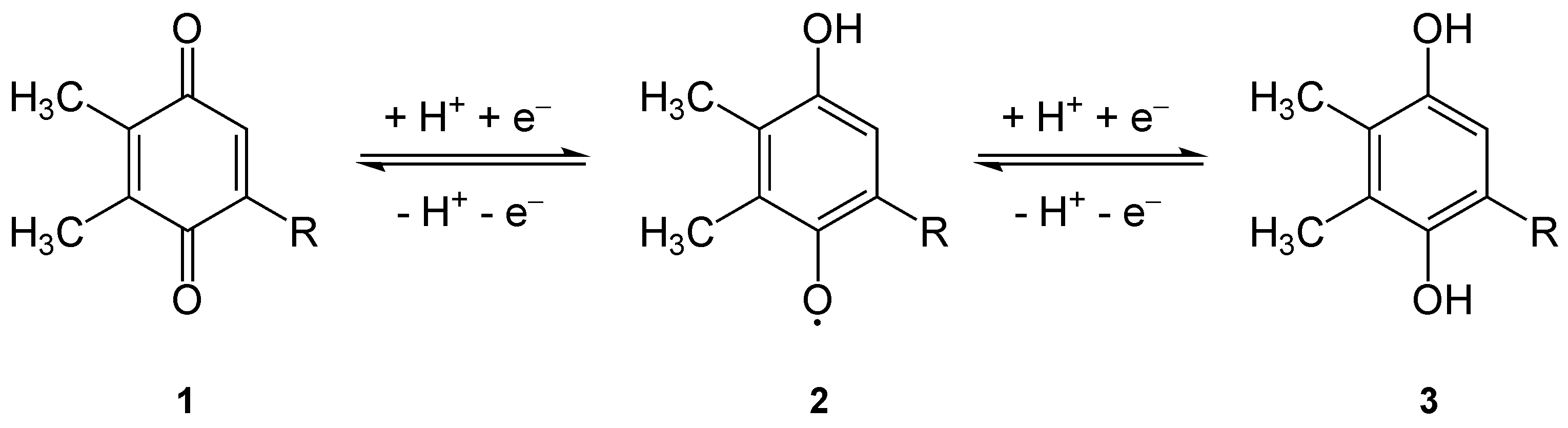
Plastoquinone (PQ) is an isoprenoid quinone molecule involved in the electron transport chain in the light-dependent reactions of photosynthesis. The most common form of plastoquinone, known as PQ-A or PQ-9, is a 2,3-dimethyl-1,4-benzoquinone molecule with a side chain of nine isoprenyl units. There are other forms of plastoquinone, such as ones with shorter side chains like PQ-3 (which has 3 isoprenyl side units instead of 9) as well as analogs such as PQ-B, PQ-C, and PQ-D, which differ in their side chains. The benzoquinone and isoprenyl units are both nonpolar, anchoring the molecule within the inner section of a lipid bilayer, where the hydrophobic tails are usually found. Plastoquinones are very structurally similar to ubiquinone, or coenzyme Q10, differing by the length of the isoprenyl side chain, replacement of the methoxy groups with methyl groups, and removal of the methyl group in the 2 position on the quinone. Like ubiquinone, it can come in several oxidation states: p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,4-Benzoquinone
1,4-Benzoquinone, commonly known as ''para''-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, being able to form oximes; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound. Preparation 1,4-Benzoquinone is prepared industrially by oxidation of hydroquinone, which can be obtained by several routes. One route involves oxidation of diisopropylbenzene and the Hock rearrangement. The net reaction can be represented as follows: :C6H4(CHMe2)2 + 3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%–6% by weight) in water for consumer use, and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or " high-test peroxide", decomposes explosively when heated and has been used as a propellant in rocketry. Hydrogen peroxide is a reactive oxygen species and the simplest peroxide, a compound having an oxygen–oxygen single bond. It decomposes slowly when exposed to light, and rapidly in the presence of organic or reactive compounds. It is typically stored with a stabilizer in a weakly acidic solution in a dark bottle to block light. Hydrogen peroxide is found in biological systems including the human body. Enzymes that use or decompose hydrogen peroxide are classified as peroxidases. Properties The boiling poi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-mevalonate Pathway
The non-mevalonate pathway—also appearing as the mevalonate-independent pathway and the 2-''C''-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate (MEP/DOXP) pathway—is an alternative metabolic pathway for the biosynthesis of the isoprenoid precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). The currently preferred name for this pathway is the MEP pathway, since MEP is the first committed metabolite on the route to IPP. Isoprenoid precursor biosynthesis The classical mevalonate pathway (MVA pathway or HMG-CoA reductase pathway) is a metabolic pathway for the biosynthesis of isoprenoid precursors: IPP and DMAPP. The MVA pathway is present in most eukaryotes and some bacteria. IPP and DMAPP serve as the basis for the biosynthesis of isoprenoid (terpenoid) molecules used in processes as diverse as protein prenylation, cell membrane maintenance, the synthesis of hormones, protein anchoring and ''N''-glycosylation in all three domains ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Greek ''tyrós'', meaning ''cheese'', as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a Hydrophobe, hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is Genetic code, encoded by the Genetic code#Codons, codons UAC and UAU in messenger RNA. Functions Aside from being a proteinogenic amino acid, tyrosine has a special role by virtue of the phenol functionality. It occurs in proteins that are part of signal transduction processes and functions as a receiver of phosphate groups that are transferred by way of protein kinases. Phosphorylation of the hyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ratio). Protons and neutrons, each with masses of approximately one atomic mass unit, are jointly referred to as "nucleons" (particles present in atomic nuclei). One or more protons are present in the nucleus of every atom. They provide the attractive electrostatic central force which binds the atomic electrons. The number of protons in the nucleus is the defining property of an element, and is referred to as the atomic number (represented by the symbol ''Z''). Since each element has a unique number of protons, each element has its own unique atomic number, which determines the number of atomic electrons and consequently the chemical characteristics of the element. The word ''proton'' is Greek for "first", and this name was given to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ATP Synthase
ATP synthase is a protein that catalyzes the formation of the energy storage molecule adenosine triphosphate (ATP) using adenosine diphosphate (ADP) and inorganic phosphate (Pi). It is classified under ligases as it changes ADP by the formation of P-O bond (phosphodiester bond). ATP synthase is a molecular machine. The overall reaction catalyzed by ATP synthase is: * ADP + Pi + 2H+out ATP + H2O + 2H+in The formation of ATP from ADP and Pi is energetically unfavorable and would normally proceed in the reverse direction. In order to drive this reaction forward, ATP synthase couples ATP synthesis during cellular respiration to an electrochemical gradient created by the difference in proton (H+) concentration across the inner mitochondrial membrane in eukaryotes or the plasma membrane in bacteria. During photosynthesis in plants, ATP is synthesized by ATP synthase using a proton gradient created in the thylakoid lumen through the thylakoid membrane and into the chloroplast stro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome B6f Complex
The cytochrome ''b''6''f'' complex (plastoquinol—plastocyanin reductase; ) is an enzyme found in the thylakoid membrane in chloroplasts of plants, cyanobacteria, and green algae, that catalyzes the transfer of electrons from plastoquinol to plastocyanin. The reaction is analogous to the reaction catalyzed by cytochrome bc1 (Complex III) of the mitochondrial electron transport chain. During photosynthesis, the cytochrome b6f complex is one step along the chain that transfers electrons from Photosystem II to Photosystem I, and at the same time pumps protons into the thylakoid space, contributing to the generation of an electrochemical (energy) gradient that is later used to synthesize ATP from ADP. Enzyme structure The cytochrome b6f complex is a dimer, with each monomer composed of eight subunits. These consist of four large subunits: a 32 kDa cytochrome f with a c-type cytochrome, a 25 kDa cytochrome b6 with a low- and high-potential heme group, a 19 kDa Rieske iron- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plastocyanin
Plastocyanin is a copper-containing protein that mediates electron-transfer. It is found in a variety of plants, where it participates in photosynthesis. The protein is a prototype of the blue copper proteins, a family of intensely blue-colored metalloproteins. Specifically, it falls into the group of small type I blue copper proteins called "cupredoxins". Function In photosynthesis, plastocyanin functions as an electron transfer agent between cytochrome f of the cytochrome ''b''6''f'' complex from photosystem II and P700+ from photosystem I. Cytochrome ''b''6''f'' complex and P700+ are both membrane-bound proteins with exposed residues on the lumen-side of the thylakoid membrane of chloroplasts. Cytochrome f acts as an electron donor while P700+ accepts electrons from reduced plastocyanin. Structure The copper site in plastocyanin, with the four amino acids that bind the metal labelled. Plastocyanin was the first of the blue copper proteins to be characterised by X-ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Transport Chain
An electron transport chain (ETC) is a series of protein complexes and other molecules that transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. The electrons that transferred from NADH and FADH2 to the ETC involves 4 multi-subunit large enzymes complexes and 2 mobile electron carriers. Many of the enzymes in the electron transport chain are membrane-bound. The flow of electrons through the electron transport chain is an exergonic process. The energy from the redox reactions creates an electrochemical proton gradient that drives the synthesis of adenosine triphosphate (ATP). In aerobic respiration, the flow of electrons terminates with molecular oxygen as the final electron acceptor. In anaerobic respiration, other electron acceptors are used, such as sulfate. In an electron transport chain, the redo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photosystem II
Photosystem II (or water-plastoquinone oxidoreductase) is the first protein complex in the light-dependent reactions of oxygenic photosynthesis. It is located in the thylakoid membrane of plants, algae, and cyanobacteria. Within the photosystem, enzymes capture photons of light to energize electrons that are then transferred through a variety of coenzymes and cofactors to reduce plastoquinone to plastoquinol. The energized electrons are replaced by oxidizing water to form hydrogen ions and molecular oxygen. By replenishing lost electrons with electrons from the splitting of water, photosystem II provides the electrons for all of photosynthesis to occur. The hydrogen ions (protons) generated by the oxidation of water help to create a proton gradient that is used by ATP synthase to generate ATP. The energized electrons transferred to plastoquinone are ultimately used to reduce to NADPH or are used in non-cyclic electron flow. DCMU is a chemical often used in laboratory sett ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thylakoid
Thylakoids are membrane-bound compartments inside chloroplasts and cyanobacteria. They are the site of the light-dependent reactions of photosynthesis. Thylakoids consist of a thylakoid membrane surrounding a thylakoid lumen. Chloroplast thylakoids frequently form stacks of disks referred to as grana (singular: granum). Grana are connected by intergranal/stromal thylakoids, which join granum stacks together as a single functional compartment. In thylakoid membranes, chlorophyll pigments are found in packets called quantasomes. Each quantasome contains 230 to 250 chlorophyll molecules. Etymology The word ''Thylakoid'' comes from the Greek word ''thylakos'' or ''θύλακος'', meaning "sac" or "pouch". Thus, ''thylakoid'' means "sac-like" or "pouch-like". Structure Thylakoids are membrane-bound structures embedded in the chloroplast stroma. A stack of thylakoids is called a granum and resembles a stack of coins. Membrane The thylakoid membrane is the site of the ligh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photosystem II - Multilingual
Photosystems are functional and structural units of protein complexes involved in photosynthesis. Together they carry out the primary photochemistry of photosynthesis: the absorption of light and the transfer of energy and electrons. Photosystems are found in the thylakoid membranes of plants, algae, and cyanobacteria. These membranes are located inside the chloroplasts of plants and algae, and in the cytoplasmic membrane of photosynthetic bacteria. There are two kinds of photosystems: PSI and PSII. PSII will absorb red light, and PSI will absorb far-red light. Although photosynthetic activity will be detected when the photosystems are exposed to either red or far-red light, the photosynthetic activity will be the greatest when plants are exposed to both wavelengths of light. Studies have actually demonstrated that the two wavelengths together have a synergistic effect on the photosynthetic activity, rather than an additive one. Each photosystem has two parts: a reaction center, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




_cyt6bf_mutant.jpg)

