|
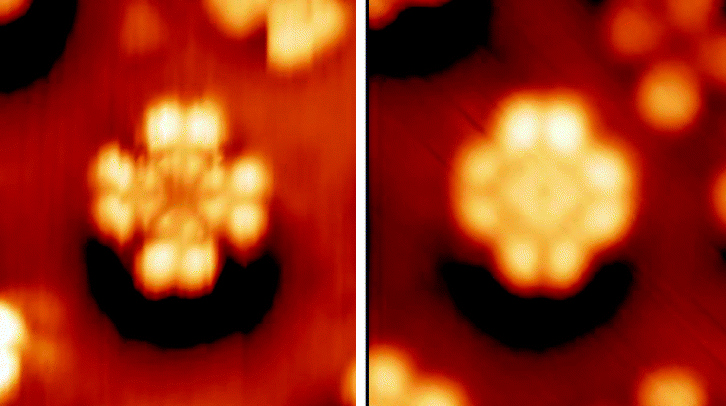
Phthalocyanine Dyes
Phthalocyanine () is a large, Aromaticity, aromatic, Macrocycle, macrocyclic, organic compound with the formula and is of theoretical or specialized interest in chemical dyes and photoelectricity. It is composed of four isoindole units linked by a ring of nitrogen atoms. = has a two-dimensional geometry and a ring system consisting of 18 π-electrons. The extensive delocalization of the π-electrons affords the molecule useful properties, lending itself to applications in dyes and pigments. Metal complexes derived from , the conjugate base of , are valuable in catalyst, catalysis, Organic solar cell, organic solar cells, and photodynamic therapy. Properties Phthalocyanine and derived metal complexes (MPc) tend to aggregate and, thus, have low solubility in common solvents. Benzene at 40 °C dissolves less than a milligram of or Phthalocyanine Blue BN, CuPc per litre. and CuPc dissolve easily in sulfuric acid due to the protonation of the nitrogen atoms bridging ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
National Institute Of Health
The National Institutes of Health, commonly referred to as NIH (with each letter pronounced individually), is the primary agency of the United States government responsible for biomedical and public health research. It was founded in the late 1880s and is now part of the United States Department of Health and Human Services. The majority of NIH facilities are located in Bethesda, Maryland, and other nearby suburbs of the Washington metropolitan area, with other primary facilities in the Research Triangle Park in North Carolina and smaller satellite facilities located around the United States. The NIH conducts its own scientific research through the NIH Intramural Research Program (IRP) and provides major biomedical research funding to non-NIH research facilities through its Extramural Research Program. , the IRP had 1,200 principal investigators and more than 4,000 postdoctoral fellows in basic, translational, and clinical research, being the largest biomedical research institut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sublimation (phase Transition)
Sublimation is the transition of a substance directly from the solid to the gas state, without passing through the liquid state. Sublimation is an endothermic process that occurs at temperatures and pressures below a substance's triple point in its phase diagram, which corresponds to the lowest pressure at which the substance can exist as a liquid. The reverse process of sublimation is deposition or desublimation, in which a substance passes directly from a gas to a solid phase. Sublimation has also been used as a generic term to describe a solid-to-gas transition (sublimation) followed by a gas-to-solid transition ( deposition). While vaporization from liquid to gas occurs as evaporation from the surface if it occurs below the boiling point of the liquid, and as boiling with formation of bubbles in the interior of the liquid if it occurs at the boiling point, there is no such distinction for the solid-to-gas transition which always occurs as sublimation from the surface. At ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serendipity
Serendipity is an unplanned fortunate discovery. Serendipity is a common occurrence throughout the history of product invention and scientific discovery. Etymology The first noted use of "serendipity" was by Horace Walpole on 28 January 1754. In a letter he wrote to his friend Horace Mann, Walpole explained an unexpected discovery he had made about a lost painting of Bianca Cappello by Giorgio Vasari by reference to a Persian fairy tale, ''The Three Princes of Serendip''. The princes, he told his correspondent, were "always making discoveries, by accidents and sagacity, of things which they were not in quest of." The name comes from ''Serendip'', an old Persian name for Sri Lanka (Ceylon), hence ''Sarandib'' by Arab traders. It is derived from the Sanskrit ''Siṃhaladvīpaḥ'' (Siṃhalaḥ, Sri Lanka + dvīpaḥ, island). The word has been exported into many other languages, with the general meaning of "unexpected discovery" or "fortunate chance". Applications Inventio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrosyl
In organic chemistry, nitroso refers to a functional group in which the nitric oxide () group is attached to an organic moiety. As such, various nitroso groups can be categorized as ''C''-nitroso compounds (e.g., nitrosoalkanes; ), ''S''-nitroso compounds ( nitrosothiols; ), ''N''-nitroso compounds (e.g., nitrosamines, ), and ''O''-nitroso compounds (alkyl nitrites; ). Synthesis Nitroso compounds can be prepared by the reduction of nitro compounds or by the oxidation of hydroxylamines. Ortho-nitrosophenols may be produced by the Baudisch reaction. In the Fischer–Hepp rearrangement aromatic 4-nitrosoanilines are prepared from the corresponding nitrosamines. Properties Nitrosoarenes typically participate in a monomer–dimer equilibrium. The dimers, which are often pale yellow, are often favored in the solid state, whereas the deep-green monomers are favored in dilute solution or at higher temperatures. They exist as ''cis'' and ''trans'' isomers. Due to the stability o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkoxy
In chemistry, the alkoxy group is an alkyl group which is singularly bonded to oxygen; thus . The range of alkoxy groups is vast, the simplest being methoxy (). An ethoxy group () is found in the organic compound ethyl phenyl ether (, also known as ethoxybenzene). Related to alkoxy groups are aryloxy groups, which have an aryl group singularly bonded to oxygen such as the phenoxy group (). An alkoxy or aryloxy group bonded to an alkyl or aryl () is an ether. If bonded to H it is an alcohol. An alkoxide can refer to salts of alcohols, and they are ionic compounds containing an alkoxide ions ; it is a derivative of an alcohol where the hydrogen of the –OH group is replaced by a metal, for example sodium salt of ethanol () is sodium ethoxide Sodium ethoxide, also referred to as sodium ethylate, is the ionic, organic compound with the formula , or NaOEt (Et = ethane). It is a white solid, although impure samples appear yellow or brown. It dissolves in polar solvents such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the "smell of natural gas" is due to the smell of the thiol used as the odorant. Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate group () bonds very strong ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as a placeholder for the aryl group in chemical structure diagrams, analogous to “R” used for any organic substituent. “Ar” is not to be confused with the elemental symbol for argon. A simple aryl group is phenyl (), a group derived from benzene. Examples of other aryl groups consist of: * The tolyl group () which is derived from toluene (methylbenzene) * The xylyl group (), which is derived from xylene (dimethylbenzene) * The naphthyl group (), which is derived from naphthalene Arylation is the process in which an aryl group is attached to a substituent. It is typically achieved by cross-coupling reactions. Nomenclature The most basic aryl group is phenyl, which is made up of a benzene ring with one hydrogen atom substituted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen. The term ''alkyl'' is intentionally unspecific to include many possible substitutions. An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalkane by removal of a hydrogen atom from a Ring (chemistry), ring and has the general formula . Typically an alkyl is a part of a larger molecule. In structural formulae, the symbol R is used to designate a generic (unspecified) alkyl group. The smallest alkyl group is methyl, with the formula . Related concepts Alkylation is an important operation in refineries, for example in the production of high-octane gasoline. Alkylating antineoplastic agents are a class of compounds that are used to treat cancer. In such case, the term alkyl is used loosely. For example, nitrogen mustards are well-known alkylating agents, but they are not simple hydrocarbons. In chemistry, alkyl is a group, a substituent, that is attached to other molecular fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy groups. Both the negatively charged anion , called hydroxide, and the neutral radical , known as the hydroxyl radical, consist of an unbonded hydroxy group. According to IUPAC definitions, the term ''hydroxyl'' refers to the hydroxyl radical () only, while the functional group is called a ''hydroxy group''. Properties Water, alcohols, carboxylic acids, and many other hydroxy-containing compounds can be readily deprotonated due to a large difference between the electronegativity of oxygen (3.5) and that of hydrogen (2.1). Hydroxy-containing compounds engage in intermolecular hydrogen bonding increasing the electrostatic attraction between molecules and thus to higher boiling and melting points than found for compounds that lack this f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is known as group 17. The word "halogen" means "salt former" (or "salt maker"). When halogens react with metals, they produce a wide range of salts, including calcium fluoride, sodium chloride (common table salt), silver bromide and potassium iodide. The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure. All of the halogens form acids when bonded to hydrogen. Most halogens are typically produced from minerals or salts. The middle halogens—chlorine, bromine, and iodine—are often used as disinfectants. Organobromides are the most important class of flame retardants, while elemental halogens are dangerous and can be toxic. History The fl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Near Infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around 1 millimeter (300 GHz) to the nominal red edge of the visible spectrum, around 700 nanometers (430 THz). Longer IR wavelengths (30 μm-100 μm) are sometimes included as part of the terahertz radiation range. Almost all black-body radiation from objects near room temperature is at infrared wavelengths. As a form of electromagnetic radiation, IR propagates energy and momentum, exerts radiation pressure, and has properties corresponding to both those of a wave and of a particle, the photon. It was long known that fires emit invisible heat; in 1681 the pioneering experimenter Edme Mariotte showed that glass, though transparent to sunlight, obstructed radiant heat. In 1800 the astronomer Sir William Herschel discovered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |