|
Photoisomerization
In chemistry, photoisomerization is a form of isomerization induced by photoexcitation. Both reversible and irreversible photoisomerizations are known for photoswitchable compounds. The term "photoisomerization" usually, however, refers to a reversible process. Applications Photoisomerization of the compound retinal in the eye allows for vision. Photoisomerizable substrates have been put to practical use, for instance, in pigments for rewritable CDs, DVDs, and 3D optical data storage solutions. In addition, interest in photoisomerizable molecules has been aimed at molecular devices, such as molecular switches, molecular motors, and molecular electronics. Another class of device that uses the photoisomerization process is as an additive in liquid crystals to change their linear and nonlinear properties. Due to the photoisomerization is possible to induce a molecular reorientation in the liquid crystal bulk, which is used in holography, as spatial filter or optical switching. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoswitch
A photoswitch is a type of molecule that can change its structural geometry and chemical properties upon irradiation with electromagnetic radiation. Although often used interchangeably with the term molecular machine, a switch does not perform work upon a change in its shape whereas a machine does. However, photochromic compounds are the necessary building blocks for light driven molecular motors and machines. Upon irradiation with light, photoisomerization about double bonds in the molecule can lead to changes in the cis- or trans- configuration. These photochromic molecules are being considered for a range of applications. Chemical structures and properties A photochromic compound can change its configuration or structure upon irradiation with light. Several examples of photochromic compounds include: azobenzene, spiropyran, merocyanine, diarylethene, spirooxazine, fulgide, hydrazone, nobormadiene, thioindigo, acrylamide-azobenzene-quaternary ammonia, donor-acceptor Stenhouse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Red
Methyl red (2-(''N'',''N''-dimethyl-4-aminophenyl) azobenzenecarboxylic acid), also called C.I. Acid Red 2, is an indicator dye that turns red in acidic solutions. It is an azo dye, and is a dark red crystalline powder. Methyl red is a pH indicator; it is red in pH under 4.4, yellow in pH over 6.2, and orange in between, with a p''K''a of 5.1. Murexide and methyl red are investigated as promising enhancers of sonochemical destruction of chlorinated hydrocarbon pollutants. Methyl red is classed by the IARC in group 3 - unclassified as to carcinogenic potential in humans. Preparation As an azo dye, methyl red may be prepared by diazotization of anthranilic acid, followed by reaction with dimethylaniline: : Properties Methyl red displays pH dependent photochromism, with protonation causing it to adopt a hydrazone/quinone structure. : Methyl Red has a special use in histopathology for showing acidic nature of tissue and presence of organisms with acidic natured cell walls. Met ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bis(triphenylphosphine)platinum Chloride
Bis(triphenylphosphine)platinum chloride is a metal phosphine complex with the formula PtCl2 (C6H5)3sub>2. Cis- and trans isomers are known. The cis isomer is a white crystalline powder, while the trans isomer is yellow. Both isomers are square planar about the central platinum atom. The cis isomer is used primarily as a reagent for the synthesis of other platinum compounds. Preparation The cis isomer is the prepared by heating solutions of platinum(II) chlorides with triphenylphosphine. For example, starting from potassium tetrachloroplatinate: :K2PtCl4 + 2 PPh3 → ''cis''-Pt(PPh3)2Cl2 + 2 KCl The trans isomer is the prepared by treating potassium trichloro(ethylene)platinate(II) (Zeise's salt) with triphenylphosphine: :KPt(C2H4)Cl3 + 2 PPh3 → ''trans''-Pt(PPh3)2Cl2 + KCl + C2H4 With heating or in the presence of excess PPh3, the trans isomer converts to the cis complex. The latter complex is the thermodynamic product due to triphenylphosphine being a strong trans effect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
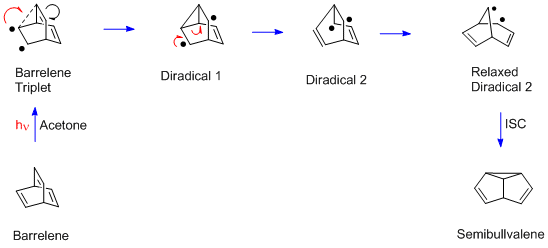
Di-π-methane Rearrangement
The di-π-methane rearrangement is a photochemical reaction of a molecular entity that contains two π-systems separated by a saturated carbon atom (a 1,4-diene or an allyl-substituted aromatic ring), to form an ene- (or aryl-) substituted cyclopropane. The rearrangement reaction formally amounts to a 1,2 shift of one ene group (in the diene) or the aryl group (in the allyl-aromatic analog) and bond ''formation'' between the lateral carbons of the non-migrating moiety. Discovery and mechanism This rearrangement was originally encountered in the photolysis of barrelene to give semibullvalene. Once the mechanism was recognized as general by Howard Zimmerman in 1967, it was clear that the structural requirement was having two pi groups attached to an sp3-hybridized carbon, and then a variety of further examples was obtained. One was the photolysis of the ''Mariano Compound'', 3,3-dimethyl-1,1,5,5-tetraphenyl-1,4-pentadiene. Another was the reaction of the ''Pratt diene'' Equat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diarylethene
Diarylethene is the general name of a class of chemical compounds that have aromatic functional groups bonded to each end of a carbon–carbon double bond. The simplest example is stilbene, which has two geometric isomers, E and Z. Under the influence of light, these compounds can generally perform two kinds of reversible isomerizations: * E to Z isomerizations, most common for stilbenes (and azobenzenes). This process goes through an excited state energy minimum where the aromatic rings lie at 90° to each other. This conformation drops to the ground state and generally relaxes to trans and cis forms in a 1:1 ratio, thus the quantum yield for E-Z isomerization is very rarely greater than 0.5. *6π electrocyclizations of the Z form, leading to an additional bond between the two aryl functionalities and a disruption of the aromatic character of these groups.J. March, ''Advanced Organic Chemistry'', 4th ed. (1992). The quantum yield of this reaction is generally less than 0.1, and in m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fulgide
In organic chemistry, a fulgide is any of a class of photochromic compounds consisting of a bis methylene-succinic anhydride core that has an aromatic group as a substituent. The highly conjugated system is a good chromophore. It can undergo reversible photoisomerization induced by ultraviolet light, converting between the ''E'' and ''Z'' isomers, both of which are typically colorless compounds. Unlike the more-stable ''Z'' isomer, the ''E'' isomer can also undergo a photochemically-induced electrocyclic reaction, forming a new ring and becoming a distinctly colored product called the ''C'' form. It is thus the two-step ''Z''–''C'' isomerization that is the photochromic change starting from the stable uncyclized form. : History The first compound of this class was synthesized in 1905, with the name based on the Latin word "fulgere", meaning shiny, for the shiny and large variety of colors of the crystal. The photochromic mechanism of fulgide was reported in 1968. Not until 1981 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of electrons as in covalent bonds. The strength of chemical bonds varies considerably; there are "strong bonds" or "primary bonds" such as covalent, ionic and metallic bonds, and "weak bonds" or "secondary bonds" such as dipole–dipole interactions, the London dispersion force and hydrogen bonding. Strong chemical bonding arises from the sharing or transfer of electrons between the participating atoms. Since opposite electric charges attract, the negatively charged electrons surrounding the nucleus and the positively charged protons within a nucleus attract each other. An electron positioned between two nuclei will be attracted to both of them, and the nuclei will be attracted toward electrons in this position. This attraction constitu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
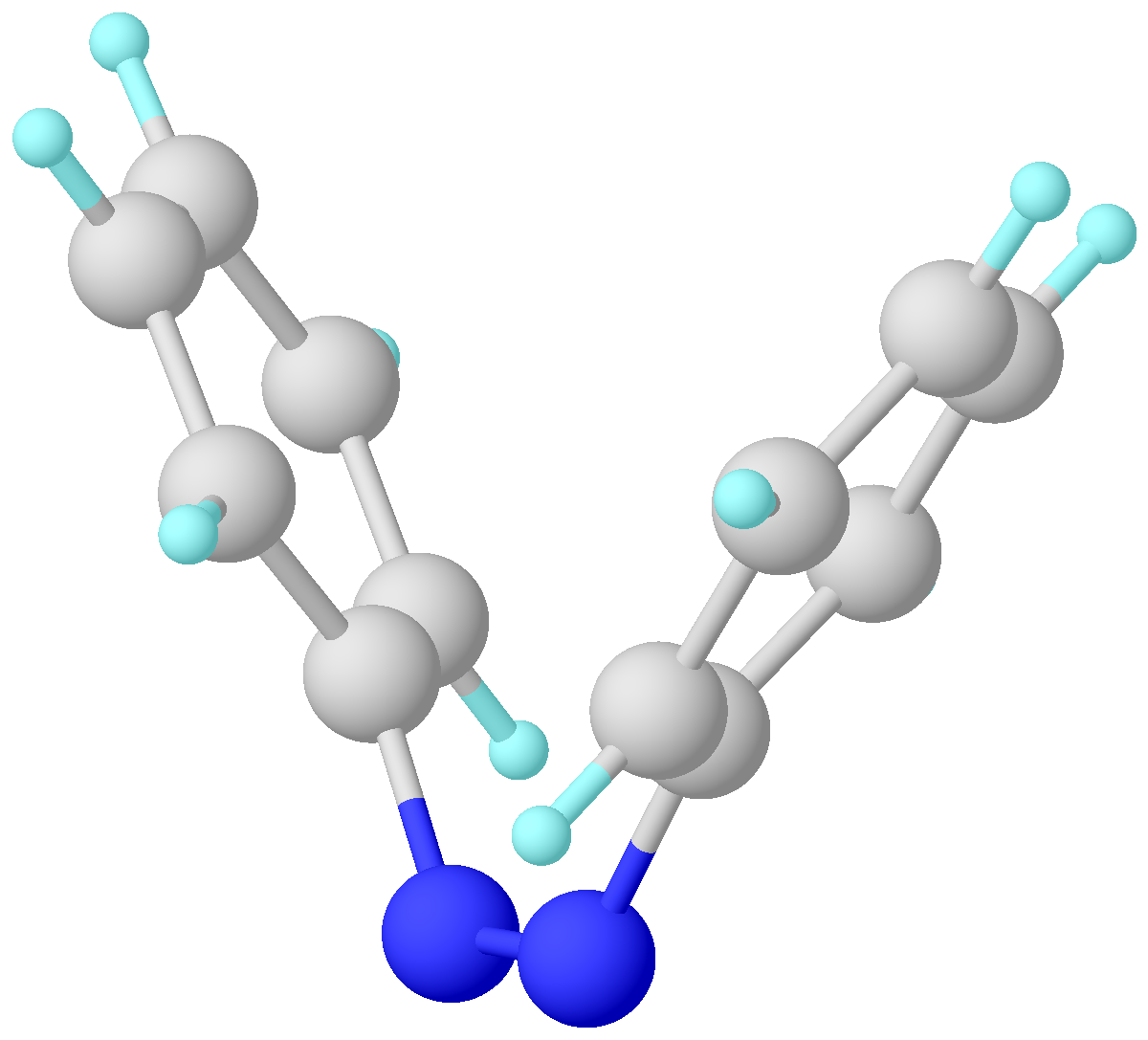
Azobenzene
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of similar compounds. These azo compounds are considered as derivatives of diazene (diimide), and are sometimes referred to as 'diazenes'. The diazenes absorb light strongly and are common dyes. Structure and synthesis ''trans''-Azobenzene is planar. The N-N distance is 1.189 Å. ''cis''-Azobenzene is nonplanar with a C-N=N-C dihedral angle of 173.5°. The N-N distance is 1.251 Å. Azobenzene was first described by Eilhard Mitscherlich in 1834. Yellowish-red crystalline flakes of azobenzene were obtained in 1856. Its original preparation is similar to the modern one. According to the 1856 method, nitrobenzene is reduced by iron filings in the presence of acetic acid. In the modern synthesis, zinc is the reductant in the presence of a base. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solar Energy
Solar energy is radiant light and heat from the Sun that is harnessed using a range of technologies such as solar power to generate electricity, solar thermal energy (including solar water heating), and solar architecture. It is an essential source of renewable energy, and its technologies are broadly characterized as either passive solar or active solar depending on how they capture and distribute solar energy or convert it into solar power. Active solar techniques include the use of photovoltaic systems, concentrated solar power, and solar water heating to harness the energy. Passive solar techniques include orienting a building to the Sun, selecting materials with favorable thermal mass or light-dispersing properties, and designing spaces that naturally circulate air. The large magnitude of solar energy available makes it a highly appealing source of electricity. In 2020 solar energy has been the cheapest source of Electricity. In Saudi Arabia a power purchase agreemen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
UV Radiation
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight, and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack the energy to ionize atoms, it can cause chemical reactions and causes many substances to glow or fluoresce. Consequently, the chemical and biological effects of UV are greater than simple heating effects, and many practical applications of UV radiation derive from its interactions with organic molecules. Short-wave ultraviolet light damages DNA and sterilizes surfaces with which it comes into contact. For h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quadricyclane
Quadricyclane is a strained, multi-cyclic hydrocarbon with the formula CH2(CH)6. A white volatile colorless liquid, it is highly strained molecule (78.7 kcal/mol). Isomerization of quadricyclane proceeds slowly at low temperatures.Petrov, V. A; Vasil’ev, N. V. “Synthetic Chemistry of Quadricyclane.” ''Current Organic Synthesis'' 3 (2006): 215–259 Because of quadricyclane’s strained structure and thermal stability, it has been studied extensively. Preparation Quadricyclane is produced by the irradiation of norbornadiene (bicyclo .2.1epta-2,5-diene) in the presence of Michler's ketone or ethyl Michler's ketone. Other sensitizers, such as acetone, benzophenone, acetophenone, etc., may be used but with a lesser yield. The yield is higher for freshly distilled norbornadiene, but commercial reagents will suffice. : Proposed applications to solar energy The conversion of norbornadiene into quadricyclane is achievd with ~300nm UV radiation. . When converted back to norbo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |