Di-π-methane Rearrangement on:
[Wikipedia]
[Google]
[Amazon]
The di-π-methane rearrangement is a
 The dependence of the success of the di-π-methane rearrangement on singlet versus triplet multiplicity arises primarily from the Free-Rotor Effect The triplet acyclic 1,4-dienes are free to undergo cis-trans interconversion of the diene double bonds (i.e. free-rotation) thus inhibiting the di-π-methane process. The cis-trans isomerization proceeds by weakening of a pi-bond and then twisting. The singlet excited states don't rotate and then are free to undergo the di-π-methane mechanism.
For cyclic dienes, as in the barrelene example, the ring structure prevents cis-trans isomerization and the di-π-methane can then occur.
The dependence of the success of the di-π-methane rearrangement on singlet versus triplet multiplicity arises primarily from the Free-Rotor Effect The triplet acyclic 1,4-dienes are free to undergo cis-trans interconversion of the diene double bonds (i.e. free-rotation) thus inhibiting the di-π-methane process. The cis-trans isomerization proceeds by weakening of a pi-bond and then twisting. The singlet excited states don't rotate and then are free to undergo the di-π-methane mechanism.
For cyclic dienes, as in the barrelene example, the ring structure prevents cis-trans isomerization and the di-π-methane can then occur.
photochemical reaction Organic photochemistry encompasses organic reactions that are induced by the action of light. The absorption of ultraviolet light by organic molecules often leads to reactions. In the earliest days, sunlight was employed, while in more modern times ...
of a molecular entity A molecular entity, or chemical entity, is "any constitutionally or isotopically distinct atom, molecule, ion, ion pair, radical, radical ion, complex, conformer, etc., identifiable as a separately distinguishable entity".{{GoldBookRef, title=molec ...
that contains two π-systems separated by a saturated carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
atom (a 1,4-diene
In organic chemistry a diene ( ) (diolefin ( ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nomenclature. ...
or an allyl-substituted aromatic ring
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
), to form an ene- (or aryl-) substituted cyclopropane. The rearrangement reaction formally amounts to a 1,2 shift of one ene group (in the diene
In organic chemistry a diene ( ) (diolefin ( ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nomenclature. ...
) or the aryl group (in the allyl-aromatic analog) and bond ''formation'' between the lateral carbons of the non-migrating moiety.
Discovery and mechanism
This rearrangement was originally encountered in the photolysis ofbarrelene
Barrelene is a bicyclic organic compound with chemical formula C8H8 and systematic name bicyclo .2.2cta-2,5,7-triene. First synthesized and described by Howard Zimmerman in 1960, the name derives from the resemblance to a barrel, with the staves ...
to give semibullvalene
Bullvalene is a hydrocarbon with the chemical formula . The molecule has a cage-like structure formed by the fusion of one cyclopropane and three cyclohepta-1,4-diene rings. Bullvalene is unusual as an organic molecule due to the and bonds fo ...
. Once the mechanism was recognized as general by Howard Zimmerman in 1967, it was clear that the structural requirement was having two pi groups attached to an sp3-hybridized carbon, and then a variety of further examples was obtained. One was the photolysis of the ''Mariano Compound'', 3,3-dimethyl-1,1,5,5-tetraphenyl-1,4-pentadiene. Another was the reaction of the ''Pratt diene''
Equation 1. The mechanism of the Mariano diene rearranging
In contrast, in the case of the Pratt diene rearranging, there are two possible regiochemistries - a and b. Process a is preferred since it leaves benzhydryl odd-electron stabilization intact.
Equation 2. The mechanism of the Pratt diene rearranging; note the regioselectivity
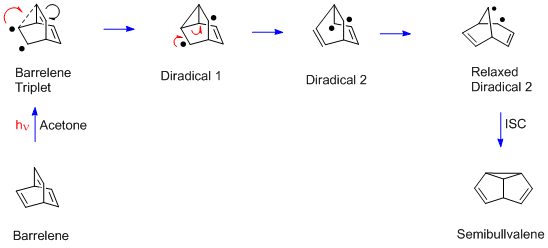
The barrelene rearrangement is now presented. It is a bit more complex than the Mariano and Pratt examples since there are two sp3-hybridized (i.e. methane) carbons. Each such bridgehead carbon has three (ethylenic) pi bonds while two are needed for the di-π-methane rearrangement. Another difference is that the barrelene reaction requires the triplet excited state while the Mariano and Pratt acyclic dienes used the excited singlet. Thus acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscib ...
is used in the barrelene reaction; acetone captures the light and then delivers triplet excitation to the barrelene reactant. In the final step of the rearrangement there is a spin-flip, termed intersystem-crossing (ISC) to provide paired electrons and a new sigma bond
In chemistry, sigma bonds (σ bonds) are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of s ...
.
Equation 3. The mechanism of the Barrelene to Semibullvalene transformation
 The dependence of the success of the di-π-methane rearrangement on singlet versus triplet multiplicity arises primarily from the Free-Rotor Effect The triplet acyclic 1,4-dienes are free to undergo cis-trans interconversion of the diene double bonds (i.e. free-rotation) thus inhibiting the di-π-methane process. The cis-trans isomerization proceeds by weakening of a pi-bond and then twisting. The singlet excited states don't rotate and then are free to undergo the di-π-methane mechanism.
For cyclic dienes, as in the barrelene example, the ring structure prevents cis-trans isomerization and the di-π-methane can then occur.
The dependence of the success of the di-π-methane rearrangement on singlet versus triplet multiplicity arises primarily from the Free-Rotor Effect The triplet acyclic 1,4-dienes are free to undergo cis-trans interconversion of the diene double bonds (i.e. free-rotation) thus inhibiting the di-π-methane process. The cis-trans isomerization proceeds by weakening of a pi-bond and then twisting. The singlet excited states don't rotate and then are free to undergo the di-π-methane mechanism.
For cyclic dienes, as in the barrelene example, the ring structure prevents cis-trans isomerization and the di-π-methane can then occur.
References
{{Reflist Rearrangement reactions