|
Photocathode
A photocathode is a surface engineered to convert light ( photons) into electrons using the photoelectric effect. Photocathodes are important in accelerator physics where they are utilised in a photoinjector to generate high brightness electron beams. Electron beams generated with photocathodes are commonly used for free electron lasers and for ultrafast electron diffraction. Photocathodes are also commonly used as the negatively charged electrode in a light detection device such as a photomultiplier or phototube. Important Properties Quantum Efficiency (QE) Quantum efficiency is a unitless number that measures the sensitivity of the photocathode to light. It is the ratio of the number of electrons emitted to the number of incident photons.Rao, T., & Dowell, D. H. (2013). ''An engineering guide to photoinjectors''. CreateSpace Independent Publishing. This property depends on the wavelength of light being used to illuminate the photocathode. For many applications, QE is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Night Vision Device
A night-vision device (NVD), also known as a night optical/observation device (NOD), night-vision goggle (NVG), is an optoelectronic device that allows visualization of images in low levels of light, improving the user's night vision. The device enhances ambient visible light and converts near-infrared light into visible light which can be seen by the user; this is known as I2 ( image intensification). By comparison, viewing of infrared thermal radiation is referred to as thermal imaging and operates in a different section of the infrared spectrum. A night vision device usually consists of an image intensifier tube, a protective housing, and may have some type of mounting system. Many NVDs also include a protective sacrificial lens, mounted over the front lens (ie. objective lens) on NVDs to protect the latter from damage by environmental hazards and some can incorporate [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Video Camera Tube
Video camera tubes were devices based on the cathode ray tube that were used in television cameras to capture television images, prior to the introduction of charge-coupled device (CCD) image sensors in the 1980s. Several different types of tubes were in use from the early 1930s, and as late as the 1990s. In these tubes, an electron beam was scanned across an image of the scene to be broadcast focused on a target. This generated a current that was dependent on the brightness of the image on the target at the scan point. The size of the striking ray was tiny compared to the size of the target, allowing 483 horizontal scan lines per image in the NTSC format, 576 lines in PAL, and as many as 1035 lines in Hi-Vision. Cathode ray tube Any vacuum tube which operates using a focused beam of electrons, originally called cathode rays, is known as a cathode ray tube (CRT). These are usually seen as display devices as used in older (i.e., non- flat panel) television receivers and compute ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoelectric Effect
The photoelectric effect is the emission of electrons when electromagnetic radiation, such as light, hits a material. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physics, and solid state and quantum chemistry to draw inferences about the properties of atoms, molecules and solids. The effect has found use in electronic devices specialized for light detection and precisely timed electron emission. The experimental results disagree with classical electromagnetism, which predicts that continuous light waves transfer energy to electrons, which would then be emitted when they accumulate enough energy. An alteration in the intensity of light would theoretically change the kinetic energy of the emitted electrons, with sufficiently dim light resulting in a delayed emission. The experimental results instead show that electrons are dislodged only when the light exceeds a certain frequency—regardless of the light's intensity o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoinjector
A photoinjector is a type of source for intense electron beams which relies on the photoelectric effect. A laser pulse incident onto the cathode of a photoinjector drives electrons out of it, and into the accelerating field of the electron gun. In comparison with the widespread thermionic electron gun, photoinjectors produce electron beams of higher brightness, which means more particles packed into smaller volume of phase space ( beam emittance). Photoinjectors serve as the main electron source for single-pass synchrotron light sources, such as free-electron lasers and for ultrafast electron diffraction setups. The first RF photoinjector was developed in 1985 at Los Alamos National Laboratory and used as the source for a free-electron-laser experiment. High-brightness electron beams produced by photoinjectors are used directly or indirectly to probe the molecular, atomic and nuclear structure of matter for fundamental research, as well as material characterization. A photoinjecto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultrafast Electron Diffraction
Ultrafast electron diffraction (UED), also known as femtosecond electron diffraction (FED), is a pump-probe experimental method based on the combination of optical pump-probe spectroscopy and electron diffraction. UED provides information on the dynamical changes of the structure of materials. It is very similar to time resolved crystallography, but instead of using X-rays as the probe, it uses electrons. In the UED technique, a femtosecond (fs) laser optical pulse excites (pumps) a sample into an excited, usually non-equilibrium, state. The pump pulse may induce chemical, electronic or structural transitions. After a finite time interval, a fs electron pulse is incident upon the sample. The electron pulse undergoes diffraction as a result of interacting with the sample. The diffraction signal is, subsequently, detected by an electron counting instrument such as a CCD camera. Specifically, after the electron pulse diffracts from the sample, the scattered electrons will form a di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vacuum Tube
A vacuum tube, electron tube, valve (British usage), or tube (North America), is a device that controls electric current flow in a high vacuum between electrodes to which an electric voltage, potential difference has been applied. The type known as a thermionic tube or thermionic valve utilizes thermionic emission of electrons from a hot cathode for fundamental electronic functions such as signal amplifier, amplification and current rectifier, rectification. Non-thermionic types such as a vacuum phototube, however, achieve electron emission through the photoelectric effect, and are used for such purposes as the detection of light intensities. In both types, the electrons are accelerated from the cathode to the anode by the electric field in the tube. The simplest vacuum tube, the diode (i.e. Fleming valve), invented in 1904 by John Ambrose Fleming, contains only a heated electron-emitting cathode and an anode. Electrons can only flow in one direction through the device—fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Image Dissector
An image dissector, also called a dissector tube, is a video camera tube in which photocathode emissions create an "electron image" which is then swept up, down and across an anode to produce an electrical signal representing the visual image. It employs magnetic fields to keep the electron image in focus, and later models used electron multiplier to pick up the electrons.Horowitz, Paul and Winfield Hill, ''The Art of Electronics'', Second Edition Cambridge University Press, 1989, pp. 1000-1001. . The term had also been used for other kinds of early video camera tubes. Dissectors were used only briefly in television systems before being replaced by the much more ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phototube
A phototube or photoelectric cell is a type of gas-filled or vacuum tube that is sensitive to light. Such a tube is more correctly called a 'photoemissive cell' to distinguish it from photovoltaic or photoconductive cells. Phototubes were previously more widely used but are now replaced in many applications by solid state photodetectors. The photomultiplier tube is one of the most sensitive light detectors, and is still widely used in physics research. Operating principles Phototubes operate according to the photoelectric effect: Incoming photons strike a photocathode, knocking electrons out of its surface, which are attracted to an anode. Thus current is dependent on the frequency and intensity of incoming photons. Unlike photomultiplier tubes, no amplification takes place, so the current through the device is typically of the order of a few microamperes. The light wavelength range over which the device is sensitive depends on the material used for the photoemissive cathod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
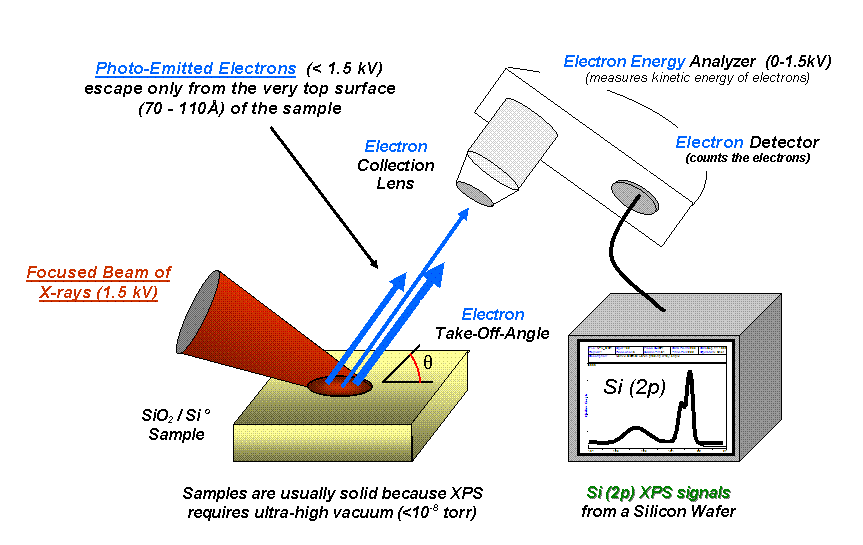
X-ray Photoelectron Spectroscopy
X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique based on the photoelectric effect that can identify the elements that exist within a material (elemental composition) or are covering its surface, as well as their chemical state, and the overall electronic structure and density of the electronic states in the material. XPS is a powerful measurement technique because it not only shows what elements are present, but also what other elements they are bonded to. The technique can be used in line profiling of the elemental composition across the surface, or in depth profiling when paired with ion-beam etching. It is often applied to study chemical processes in the materials in their as-received state or after cleavage, scraping, exposure to heat, reactive gasses or solutions, ultraviolet light, or during ion implantation. XPS belongs to the family of photoemission spectroscopies in which electron population spectra are obtained by irr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali Metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names for the elements in some languages, such as German and Russian. rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour. This family of elements is also known as the lithium family after its leading element. The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and readil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimony
Antimony is a chemical element with the symbol Sb (from la, stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient times and were powdered for use as medicine and cosmetics, often known by the Arabic name kohl. The earliest known description of the metal in the West was written in 1540 by Vannoccio Biringuccio. China is the largest producer of antimony and its compounds, with most production coming from the Xikuangshan Mine in Hunan. The industrial methods for refining antimony from stibnite are roasting followed by reduction with carbon, or direct reduction of stibnite with iron. The largest applications for metallic antimony are in alloys with lead and tin, which have improved properties for solders, bullets, and plain bearings. It improves the rigidity of lead-alloy plates in lead–acid batteries. Antimony trioxide is a prominent additive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |