|
Photocatalyst
In chemistry, photocatalysis is the acceleration of a photoreaction in the presence of a catalyst. In catalyzed photolysis, light is absorbed by an adsorbed substrate. In photogenerated catalysis, the photocatalytic activity depends on the ability of the catalyst to create electron–hole pairs, which generate free radicals (e.g. hydroxyl radicals: •OH) able to undergo secondary reactions. Its practical application was made possible by the discovery of water electrolysis by means of titanium dioxide (). History Early mentions (1911–1938) The earliest mention came in 1911, when German chemist Dr. Alexander Eibner integrated the concept in his research of the illumination of zinc oxide (ZnO) on the bleaching of the dark blue pigment, Prussian blue. Around this time, Bruner and Kozak published an article discussing the deterioration of oxalic acid in the presence of uranyl salts under illumination, while in 1913, Landau published an article explaining the phenomenon of p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Dioxide
Titanium dioxide, also known as titanium(IV) oxide or titania , is the inorganic compound with the chemical formula . When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or CI 77891. It is a white solid that is insoluble to water, although mineral forms can appear black. As a pigment, it has a wide range of applications, including paint, sunscreen, and food coloring. When used as a food coloring, it has E number E171. World production in 2014 exceeded 9 million tonnes. It has been estimated that titanium dioxide is used in two-thirds of all pigments, and pigments based on the oxide have been valued at a price of $13.2 billion. Structure In all three of its main dioxides, titanium exhibits octahedral geometry, being bonded to six oxide anions. The oxides in turn are bonded to three Ti centers. The overall crystal structure of rutile is tetragonal in symmetry whereas anatase and brookite are orthorhombic. The oxygen substructures are all slight dist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photooxidation
In polymer chemistry photo-oxidation (sometimes: oxidative photodegradation) is the degradation of a polymer surface due to the combined action of light and oxygen. It is the most significant factor in the weathering of plastics. Photo-oxidation causes the polymer chains to break (chain scission), resulting in the material becoming increasingly brittle. This leads to mechanical failure and, at an advanced stage, the formation of microplastics. In textiles the process is called phototendering. Technologies have been developed to both accelerate and inhibit this process. For example, plastic building components like doors, window frames and gutters are expected to last for decades, requiring the use of advanced UV- polymer stabilizers. Conversely, single-use plastics can be treated with biodegradable additives to accelerate their fragmentation. Many pigments and dyes can similarly have effects due to their ability to absorb UV-energy. Susceptible polymers Susceptibility to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
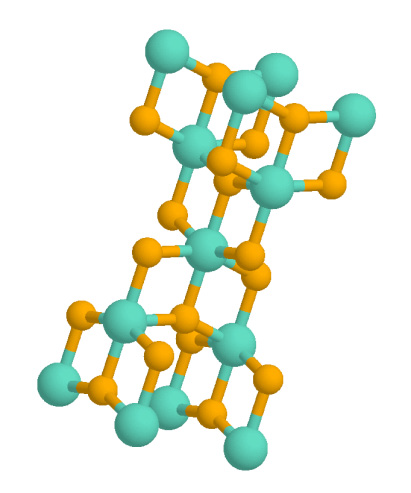
Anatase (titania, TiO2) Photocatalyst Producing Hydrogen
Anatase is a metastable mineral form of titanium dioxide (TiO2) with a tetragonal crystal structure. Although colorless or white when pure, anatase in nature is usually a black solid due to impurities. Three other polymorphs (or mineral forms) of titanium dioxide are known to occur naturally: brookite, akaogiite, and rutile, with rutile being the most common and most stable of the bunch. Anatase is formed at relatively low temperatures and found in minor concentrations in igneous and metamorphic rocks. Thin films of TiO2-coated glass show antifogging and self-cleaning properties under ultraviolet radiation. Anatase is always found as small, isolated, and sharply developed crystals, and like rutile, it crystallizes in a tetragonal system. Anatase is metastable at all temperatures and pressures, with rutile being the equilibrium polymorph. Nevertheless, anatase is often the first titanium dioxide phase to form in many processes due to its lower surface energy, with a transfo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formaldehyde
Formaldehyde ( , ) ( systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section Forms below), hence it is stored as an aqueous solution (formalin), which is also used to store animal specimens. It is the simplest of the aldehydes (). The common name of this substance comes from its similarity and relation to formic acid. Formaldehyde is an important precursor to many other materials and chemical compounds. In 1996, the installed capacity for the production of formaldehyde was estimated at 8.7 million tons per year. It is mainly used in the production of industrial resins, e.g., for particle board and coatings. Forms Formaldehyde is more complicated than many simple carbon compounds in that it adopts several diverse forms. These compounds can often be used interchangeably and can be interconverted. *Molecular for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile metal in a pure form. Chemically, gold is a transition metal and a group 11 element. It is one of the least reactive chemical elements and is solid under standard conditions. Gold often occurs in free elemental (native state), as nuggets or grains, in rocks, veins, and alluvial deposits. It occurs in a solid solution series with the native element silver (as electrum), naturally alloyed with other metals like copper and palladium, and mineral inclusions such as within pyrite. Less commonly, it occurs in minerals as gold compounds, often with tellurium ( gold tellurides). Gold is resistant to most acids, though it does dissolve in aqua regia (a mixture of nitric acid and hydrochloric acid), forming a soluble tetrachloroau ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gasification
Gasification is a process that converts biomass- or fossil fuel-based carbonaceous materials into gases, including as the largest fractions: nitrogen (N2), carbon monoxide (CO), hydrogen (H2), and carbon dioxide (). This is achieved by reacting the feedstock material at high temperatures (typically >700 °C), without combustion, via controlling the amount of oxygen and/or steam present in the reaction. The resulting gas mixture is called syngas (from synthesis gas) or producer gas and is itself a fuel due to the flammability of the H2 and CO of which the gas is largely composed. Power can be derived from the subsequent combustion of the resultant gas, and is considered to be a source of renewable energy if the gasified compounds were obtained from biomass feedstock. An advantage of gasification is that syngas can be more efficient than direct combustion of the original feedstock material because it can be combusted at higher temperatures so that the thermodynamic upper ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cracking (chemistry)
In petrochemistry, petroleum geology and organic chemistry, cracking is the process whereby complex organic molecules such as kerogens or long-chain hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of carbon-carbon bonds in the precursors. The rate of cracking and the end products are strongly dependent on the temperature and presence of catalysts. Cracking is the breakdown of a large alkane into smaller, more useful alkenes. Simply put, hydrocarbon cracking is the process of breaking a long chain of hydrocarbons into short ones. This process requires high temperatures. More loosely, outside the field of petroleum chemistry, the term "cracking" is used to describe any type of splitting of molecules under the influence of heat, catalysts and solvents, such as in processes of destructive distillation or pyrolysis. Fluid catalytic cracking produces a high yield of petrol and LPG, while hydrocracking is a major source of jet fuel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver". Platinum is a member of the platinum group of elements and group 10 of the periodic table of elements. It has six naturally occurring isotopes. It is one of the rarer elements in Earth's crust, with an average abundance of approximately 5 μg/kg. It occurs in some nickel and copper ores along with some native deposits, mostly in South Africa, which accounts for ~80% of the world production. Because of its scarcity in Earth's crust, only a few hundred tonnes are produced annually, and given its important uses, it is highly valuable and is a major precious metal commodity. Platinum is one of the least reactive metals. It has remarkable resistance to corrosion, even at high temperatures, and is therefore considered a noble metal. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photodissociation
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. Photodissociation is not limited to visible light. Any photon with sufficient energy can affect the chemical bonds of a chemical compound. Since a photon's energy is inversely proportional to its wavelength, electromagnetic radiations with the energy of visible light or higher, such as ultraviolet light, x-rays, and gamma rays can induce such reactions. Photolysis in photosynthesis Photolysis is part of the light-dependent reaction or light phase or photochemical phase or Hill reaction of photosynthesis. The general reaction of photosynthetic photolysis can be given in terms of photons as: :\ce + 2 \text \longrightarrow \ce The chemical nature of "A" depends on the type of organism. Purple sulfur bacteria oxidize hydrogen sulfide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kenichi Honda
Kenichi Honda (本多健一, August 23, 1925 – February 26, 2011) was a Japanese chemist. He made a significant contribution to the discovery and characterization of photocatalytic properties of titanium dioxide (TiO2), for which he shared the 2004 Japan Prize with his former student Akira Fujishima. Biography In 1949 Honda received his bachelor's degree in engineering from the University of Tokyo. He then spent several years in France, where he defended a PhD in 1957 at the University of Paris. After returning to Japan he obtained a second doctorate degree, from the University of Tokyo in 1961. He then worked as a lecturer (1965–1975) and professor (1975–1983) at the University of Tokyo and at Kyoto University (1983–1989). In 1989 he moved to the Tokyo Polytechnic University and served as its president in 1996–2004. Research In the late 1960s Honda and his doctorate student Akira Fujishima discovered the Honda-Fujishima effect - photocatalytic water decomposition (pho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Akira Fujishima
is a Japanese chemist and president of Tokyo University of Science. He is known for significant contributions to the discovery and research of photocatalytic and superhydrophilic properties of titanium dioxide (TiO2), which is also known as the ''Honda-Fujishima effect''.(Originally published in Japanese 2 December 2003) Career and research In 1966 he earned his B. A. (Engineering) at the Faculty of Engineering, Yokohama National University, and in 1971 his Ph.D. (Engineering) at the Graduate School of Engineering, the University of Tokyo. In 1967, while working on his Ph.D. under the supervision of professor Kenichi Honda (本多 健一), he discovered the phenomenon of photocatalytic water decomposition (water photolysis) when he exposed a titanium dioxide electrode to strong light, later called the ''Honda-Fujishima effect''. The discovery of self-cleaning properties of titanium dioxide by the group under his supervision initiated a revolution in the ceramic, glass, and ot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_photocatalyst_producing_hydrogen.jpg)