|
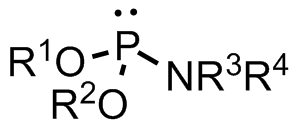
Phosphorodiamidate
Phosphoramidates (sometimes also called amidophosphates) are a class of phosphorus compounds structurally related to phosphates (or organophosphates) via the substitution of an OR for a NR2. They are derivatives of phosphoramidic acids O=P(OH)(NR2)2, O=P(OH)2(NR2). A phosphorodiamidate (or diamidophosphate) is a phosphate that has two of its OH groups substituted by NR2 groups to give a species with the general formula O=P(OH)(NH2)2. The substitution of all three OH groups gives the phosphoric triamides (O=P(NR2)3), which are commonly referred to as phosphoramides. Examples Two examples of natural phosphoramidates are phosphocreatine and the phosphoramidate formed when histidine residues in histidine kinases are phosphorylated. An example of a phosphorodiamidate is morpholino which is used in molecular biology. See also *Phosphoramidite A phosphoramidite (RO)2PNR2 is a monoamide of a phosphite diester. The key feature of phosphoramidites is their markedly high reactivity to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fosthietan
Fosthietan (chemical formula:) is a chemical compound used in insecticides and nematicide A nematicide is a type of chemical pesticide used to kill plant-parasitic nematodes. Nematicides have tended to be broad-spectrum toxicants possessing high volatility or other properties promoting migration through the soil. Aldicarb (Temik), a ...s. References Insecticides Phosphoramidates Dithietanes Ethyl esters Nematicides {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Earth. It has a concentration in the Earth's crust of about one gram per kilogram (compare copper at about 0.06 grams). In minerals, phosphorus generally occurs as phosphate. Elemental phosphorus was first isolated as white phosphorus in 1669. White phosphorus emits a faint glow when exposed to oxygen – hence the name, taken from Greek mythology, meaning 'light-bearer' (Latin ), referring to the "Morning Star", the planet Venus. The term '' phosphorescence'', meaning glow after illumination, derives from this property of phosphorus, although the word has since been used for a different physical process that produces a glow. The glow of phosphorus is caused by oxidation of the white (but not red) phosphorus — a process now called chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid . The phosphate or orthophosphate ion is derived from phosphoric acid by the removal of three protons . Removal of one or two protons gives the dihydrogen phosphate ion and the hydrogen phosphate ion ion, respectively. These names are also used for salts of those anions, such as ammonium dihydrogen phosphate and trisodium phosphate. File:3-phosphoric-acid-3D-balls.png, Phosphoricacid File:2-dihydrogenphosphate-3D-balls.png, Dihydrogenphosphate File:1-hydrogenphosphate-3D-balls.png, Hydrogenphosphate File:0-phosphate-3D-balls.png, Phosphate In organic chemistry, phosphate or orthophosphate is an organophosphate, an ester of orthophosphoric acid of the form where one or more hydrogen atoms are replaced by organic groups. An example is trimethyl phosphate, . The term also refers to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphate
In organic chemistry, organophosphates (also known as phosphate esters, or OPEs) are a class of organophosphorus compounds with the general structure , a central phosphate molecule with alkyl or aromatic substituents. They can be considered as esters of phosphoric acid. Like most functional groups, organophosphates occur in a diverse range of forms, with important examples including key biomolecules such as DNA, RNA and ATP, as well as many insecticides, herbicides, nerve agents and flame retardants. OPEs have been widely used in various products as flame retardants, plasticizers, and performance additives to engine oil. The popularity of OPEs as flame retardants came as a substitution for the highly regulated brominated flame retardants. The low cost of production and compatibility to diverse polymers made OPEs to be widely used in industry including textile, furniture, electronics as plasticizers and flame retardants. These compounds are added to the final product phys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamidophosphate
Diamidophosphate (DAP) is the simplest phosphorodiamidate ion, with formula PO2(NH2)2−. It is a phosphorylating ion and was first used for phosphorylation of sugars in aqueous medium. DAP has attracted interest in the area of primordial chemistry. Salts Several salts of the formula MPO2(NH2)2(H2O)x are known. *The sodium salt can be made by base hydrolysis of phenyl phosphorodiamidate. It crystallises as a hexahydrate. It can be dehydrated. *The silver salt AgPO2(NH2)2 can react using double decomposition with bromides forming other salts. *The potassium diamidophosphate salt KPO2(NH2)2 is also known. *Phosphorodiamidic acid crystallizes as a trihydrate. Reactions Heating anhydrous sodium diamidophosphate causes polmerization: *At 160 °C, Na2P2O4(NH)(NH2)2, Na3P3O6(NH)2(NH2)2, Na4P4O8(NH)3(NH2)2, Na5P5O10(NH)4(NH2)2 and Na6P6O12(NH)5(NH2)2 are produced. These substances contain P-N-P backbones. These can be separated by paper chromatography. *At 200 °C the hexa- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoramides
Phosphoramides are a class of phosphorus compounds with the formula O=P(NR2)3-n(OH)n. They can be considered derivatives of phosphoric acid where OH groups have been replaced with an amino or R-substituted amino group. In practise the term is commonly confined to the phosphoric triamides (P(=O)(NR2)3), essentially phosphoramide and derivatives thereof. Derivatives with the general structures P(=O)(OH)(NR2)2 or P(=O)(OH)2(NR2) are usually referred to as phosphoramidic acids. Examples *Phenyl phosphorodiamidate, a phosphoramide but also a phosphate ester, is used in agriculture to enhance the effectiveness of urea-based fertilizers. *Hexamethylphosphoramide Hexamethylphosphoramide, often abbreviated HMPA, is a phosphoramide (an amide of phosphoric acid) with the formula This colorless liquid is a useful reagent in organic synthesis. Structure and reactivity HMPA is the oxide of the highly basic t ... (HMPA) is a polar solvent. References {{Organophosphorus Functiona ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphocreatine
Phosphocreatine, also known as creatine phosphate (CP) or PCr (Pcr), is a phosphorylated form of creatine that serves as a rapidly mobilizable reserve of high-energy phosphates in skeletal muscle, myocardium and the brain to recycle adenosine triphosphate, the energy currency of the cell. Chemistry In the kidneys, the enzyme AGAT catalyzes the conversion of two amino acids — arginine and glycine — into guanidinoacetate (also called glycocyamine or GAA), which is then transported in the blood to the liver. A methyl group is added to GAA from the amino acid methionine by the enzyme GAMT, forming non-phosphorylated creatine. This is then released into the blood by the liver where it travels mainly to the muscle cells (95% of the body's creatine is in muscles), and to a lesser extent the brain, heart, and pancreas. Once inside the cells it is transformed into phosphocreatine by the enzyme complex creatine kinase. Phosphocreatine is able to donate its phosphate group to convert ad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histidine Kinase
Histidine kinases (HK) are multifunctional, and in non-animal kingdoms, typically transmembrane, proteins of the transferase class of enzymes that play a role in signal transduction across the cellular membrane. The vast majority of HKs are homodimers that exhibit autokinase, phosphotransfer, and phosphatase activity. HKs can act as cellular receptors for signaling molecules in a way analogous to tyrosine kinase receptors (RTK). Multifunctional receptor molecules such as HKs and RTKs typically have portions on the outside of the cell (extracellular domain) that bind to hormone- or growth factor-like molecules, portions that span the cell membrane (transmembrane domain), and portions within the cell (intracellular domain) that contain the enzymatic activity. In addition to kinase activity, the intracellular domains typically have regions that bind to a secondary effector molecule or complex of molecules that further propagate signal transduction within the cell. Distinct from other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Morpholino
A Morpholino, also known as a Morpholino oligomer and as a phosphorodiamidate Morpholino oligomer (PMO), is a type of oligomer molecule (colloquially, an oligo) used in molecular biology to modify gene expression. Its molecular structure contains DNA bases attached to a backbone of methylenemorpholine rings linked through phosphorodiamidate groups. Morpholinos block access of other molecules to small (~25 base) specific sequences of the base-pairing surfaces of ribonucleic acid (RNA). Morpholinos are used as research tools for reverse genetics by knocking down gene function. This article discusses only the Morpholino antisense oligomers, which are nucleic acid analogs. The word "Morpholino" can occur in other chemical names, referring to chemicals containing a six-membered morpholine ring. To help avoid confusion with other morpholine-containing molecules, when describing oligos "Morpholino" is often capitalized as a trade name, but this usage is not consistent across scientifi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Biology
Molecular biology is the branch of biology that seeks to understand the molecular basis of biological activity in and between cells, including biomolecular synthesis, modification, mechanisms, and interactions. The study of chemical and physical structure of biological macromolecules is known as molecular biology. Molecular biology was first described as an approach focused on the underpinnings of biological phenomena - uncovering the structures of biological molecules as well as their interactions, and how these interactions explain observations of classical biology. In 1945 the term molecular biology was used by physicist William Astbury. In 1953 Francis Crick, James Watson, Rosalind Franklin, and colleagues, working at Medical Research Council unit, Cavendish laboratory, Cambridge (now the MRC Laboratory of Molecular Biology), made a double helix model of DNA which changed the entire research scenario. They proposed the DNA structure based on previous research done by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoramidite
A phosphoramidite (RO)2PNR2 is a monoamide of a phosphite diester. The key feature of phosphoramidites is their markedly high reactivity towards nucleophiles catalyzed by weak acids ''e.c''., triethylammonium chloride or 1''H''-tetrazole. In these reactions, the incoming nucleophile replaces the NR2 moiety. Applications Nucleoside phosphoramidites Phosphoramidites derived from protected nucleosides are referred to as nucleoside phosphoramidites and are widely used in chemical synthesis of DNA, RNA, and other nucleic acids and their analogs. As ligands Certain phosphoramidites are also used as monodentate chiral ligands in asymmetric synthesis. A large group of such ligands is derived from the chiral diol BINOL and can be synthesised by reaction of BINOL with phosphorus trichloride to the chlorophosphite and then reaction with simple secondary amine In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |