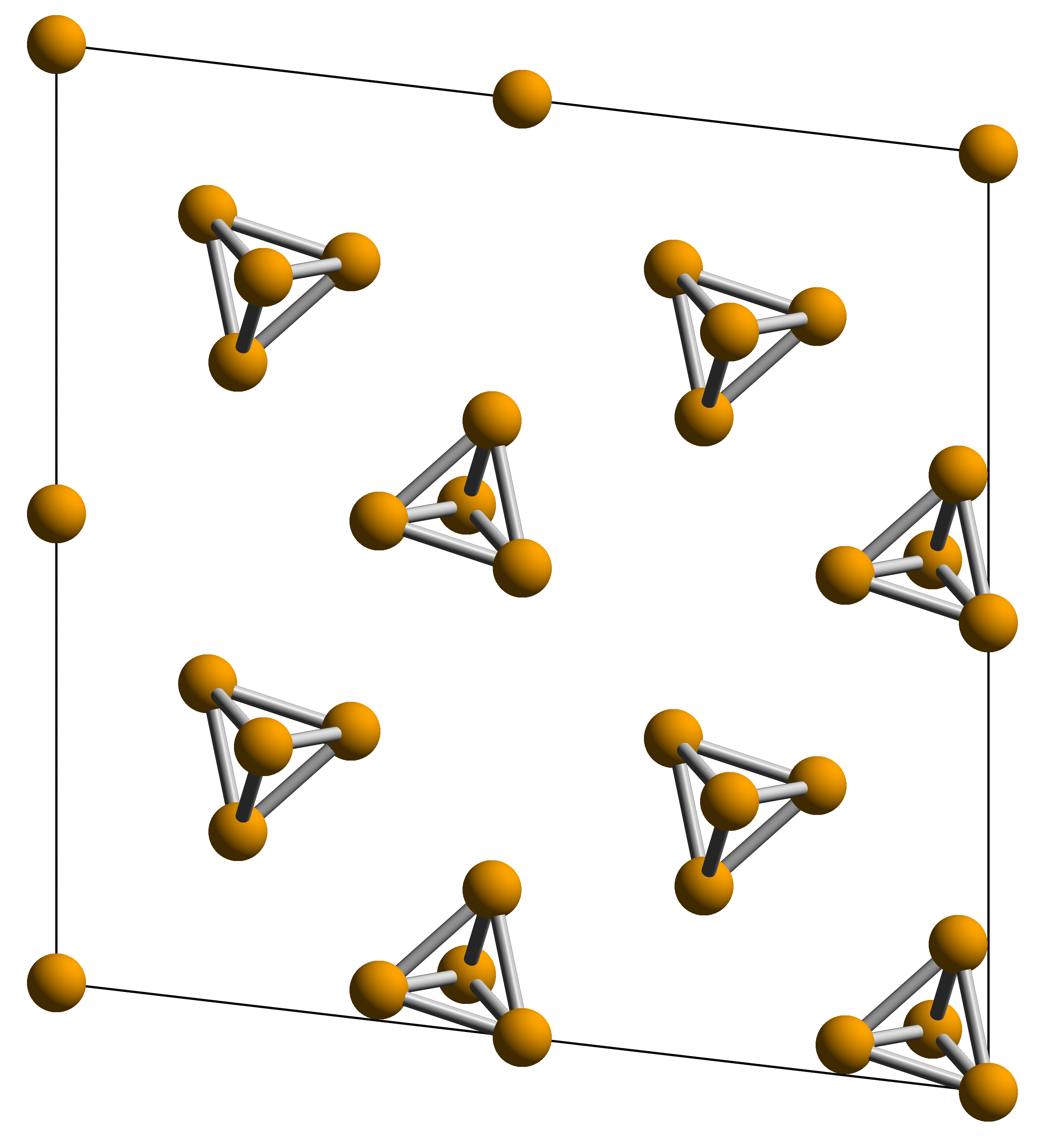|
Phospho
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Earth. It has a concentration in the Earth's crust of about one gram per kilogram (compare copper at about 0.06 grams). In minerals, phosphorus generally occurs as phosphate. Elemental phosphorus was first isolated as white phosphorus in 1669. White phosphorus emits a faint glow when exposed to oxygen – hence the name, taken from Greek mythology, meaning 'light-bearer' (Latin ), referring to the " Morning Star", the planet Venus. The term ''phosphorescence'', meaning glow after illumination, derives from this property of phosphorus, although the word has since been used for a different physical process that produces a glow. The glow of phosphorus is caused by oxidation of the white (but not red) phosphorus — a process now called chemilumi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
White Phosphorus
Elemental phosphorus can exist in several allotropes, the most common of which are white and red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists as diphosphorus and atomic phosphorus. White phosphorus White phosphorus, yellow phosphorus or simply tetraphosphorus () exists as molecules made up of four atoms in a tetrahedral structure. The tetrahedral arrangement results in ring strain and instability. The molecule is described as consisting of six single P–P bonds. Two crystalline forms are known. The α form is defined as the standard state of the element, but is actually metastable under standard conditions. It has a body-centered cubic crystal structure, and transforms reversibly into the β form at 195.2 K. The β form is believed to have a hexagonal crystal structure. White phosphorus is a translucent waxy solid that quickly becomes yellow when exposed to light. For this reason it is also called yellow phosphorus. It glows greenis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Red Phosphorus
Elemental phosphorus can exist in several allotropes, the most common of which are white and red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists as diphosphorus and atomic phosphorus. White phosphorus White phosphorus, yellow phosphorus or simply tetraphosphorus () exists as molecules made up of four atoms in a tetrahedral structure. The tetrahedral arrangement results in ring strain and instability. The molecule is described as consisting of six single P–P bonds. Two crystalline forms are known. The α form is defined as the standard state of the element, but is actually metastable under standard conditions. It has a body-centered cubic crystal structure, and transforms reversibly into the β form at 195.2 K. The β form is believed to have a hexagonal crystal structure. White phosphorus is a translucent waxy solid that quickly becomes yellow when exposed to light. For this reason it is also called yellow phosphorus. It glows greenish in t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allotropes Of Phosphorus
Elemental phosphorus can exist in several allotropes, the most common of which are white and red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists as diphosphorus and atomic phosphorus. White phosphorus White phosphorus, yellow phosphorus or simply tetraphosphorus () exists as molecules made up of four atoms in a tetrahedral structure. The tetrahedral arrangement results in ring strain and instability. The molecule is described as consisting of six single P–P bonds. Two crystalline forms are known. The α form is defined as the standard state of the element, but is actually metastable under standard conditions. It has a body-centered cubic crystal structure, and transforms reversibly into the β form at 195.2 K. The β form is believed to have a hexagonal crystal structure. White phosphorus is a translucent waxy solid that quickly becomes yellow when exposed to light. For this reason it is also called yellow phosphorus. It glows greenis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorescence
Phosphorescence is a type of photoluminescence related to fluorescence. When exposed to light (radiation) of a shorter wavelength, a phosphorescent substance will glow, absorbing the light and reemitting it at a longer wavelength. Unlike fluorescence, a phosphorescent material does not immediately reemit the radiation it absorbs. Instead, a phosphorescent material absorbs some of the radiation energy and reemits it for a much longer time after the radiation source is removed. In a general sense, there is no distinct boundary between the emission times of fluorescence and phosphorescence (i.e.: if a substance glows under a black light it is generally considered fluorescent, and if it glows in the dark it is often simply called phosphorescent). In a modern, scientific sense, the phenomena can usually be classified by the three different mechanisms that produce the light, and the typical timescales during which those mechanisms emit light. Whereas fluorescent materials stop emitt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phospholipid
Phospholipids, are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typically have omega-3 fatty acids EPA and DHA integrated as part of the phospholipid molecule. The phosphate group can be modified with simple organic molecules such as choline, ethanolamine or serine. Phospholipids are a key component of all cell membranes. They can form lipid bilayers because of their amphiphilic characteristic. In eukaryotes, cell membranes also contain another class of lipid, sterol, interspersed among the phospholipids. The combination provides fluidity in two dimensions combined with mechanical strength against rupture. Purified phospholipids are produced commercially and have found applications in nanotechnology and materials science. The first phospholipid identified in 1847 as such in biological tissues wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pnictogen
A pnictogen ( or ; from grc, πνῑ́γω "to choke" and -gen, "generator") is any of the chemical elements in group 15 of the periodic table. Group 15 is also known as the nitrogen group or nitrogen family. Group 15 consists of the elements nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), bismuth (Bi), and moscovium (Mc). Since 1988, IUPAC calls it Group 15. Before that, in America it was called Group VA, owing to a text by H. C. Deming and the Sargent-Welch Scientific Company, while in Europe it was called Group VB and IUPAC recommended that in 1970. (Pronounced "group five A" and "group five B"; "V" is the Roman numeral 5). In semiconductor physics, it is still usually called Group V. The "five" ("V") in the historical names comes from the " pentavalency" of nitrogen, reflected by the stoichiometry of compounds such as N2O5. They have also been called the pentels. Characteristics Chemical Like other groups, the members of this family show similar pat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphorus Compound
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents. Organophosphorus chemistry is the corresponding science of the properties and reactivity of organophosphorus compounds. Phosphorus, like nitrogen, is in group 15 of the periodic table, and thus phosphorus compounds and nitrogen compounds have many similar properties. The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct phosphorus-carbon (P-C) bond. Thus a large proportion of pesticides (e.g., malathion), are often included in this class of compounds. Phosphorus can adopt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus (morning Star)
Phosphorus () is one of the '' Astra Planeta'', specifically the god of the planet Venus in its appearance as the Morning Star. Another Greek name for the Morning Star is "Eosphorus" ( grc, Ἑωσφόρος, Heōsphoros, link=no), which means "dawn-bringer". The term "eosphorus" is sometimes met in English. As an adjective, the word "phosphorus" is applied in the sense of "light-bringing" (for instance, the dawn, the god Dionysus, pine torches and the day) and "torch-bearing" as an epithet of several gods and goddesses, especially of Hecate but also of Artemis/Diana and Hephaestus. Seasonally, Venus is the "light bringer" in the northern hemisphere, appearing most brightly in December (an optical illusion due to shorter days), signalling the "rebirth" of longer days as winter wanes. Venus The morning star is an appearance of the planet Venus, an inferior planet, meaning that its orbit lies between that of the Earth and the Sun. Depending on the orbital locations of both Ven ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid . The phosphate or orthophosphate ion is derived from phosphoric acid by the removal of three protons . Removal of one or two protons gives the dihydrogen phosphate ion and the hydrogen phosphate ion ion, respectively. These names are also used for salts of those anions, such as ammonium dihydrogen phosphate and trisodium phosphate. File:3-phosphoric-acid-3D-balls.png, Phosphoricacid File:2-dihydrogenphosphate-3D-balls.png, Dihydrogenphosphate File:1-hydrogenphosphate-3D-balls.png, Hydrogenphosphate File:0-phosphate-3D-balls.png, Phosphate In organic chemistry, phosphate or orthophosphate is an organophosphate, an ester of orthophosphoric acid of the form where one or more hydrogen atoms are replaced by organic groups. An example is trimethyl phosphate, . The term also refers to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lucifer
Lucifer is one of various figures in folklore associated with the planet Venus. The entity's name was subsequently absorbed into Christianity as a name for the devil. Modern scholarship generally translates the term in the relevant Bible passage ( Isaiah 14:12), where the Greek Septuagint reads ὁ ἑωσφόρος ὁ πρωὶ, as "morning star" or "shining one" rather than as a proper noun, Lucifer, as found in the Latin Vulgate. As a name for the Devil in Christian theology, the more common meaning in English, "Lucifer" is the rendering of the Hebrew word he, הֵילֵל, hêlēl, label=none, (pronunciation: ''hay-lale'') in Isaiah given in the King James Version of the Bible. The translators of this version took the word from the Latin Vulgate, Originally published New York: The MacMillan Co., 1923. which translated by the Latin word (uncapitalized), meaning "the morning star", "the planet Venus", or, as an adjective, "light-bringing". As a name for the planet in its ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fertiliser
A fertilizer (American English) or fertiliser (British English; see spelling differences) is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from liming materials or other non-nutrient soil amendments. Many sources of fertilizer exist, both natural and industrially produced. For most modern agricultural practices, fertilization focuses on three main macro nutrients: nitrogen (N), phosphorus (P), and potassium (K) with occasional addition of supplements like rock flour for micronutrients. Farmers apply these fertilizers in a variety of ways: through dry or pelletized or liquid application processes, using large agricultural equipment or hand-tool methods. Historically fertilization came from natural or organic sources: compost, animal manure, human manure, harvested minerals, crop rotations and byproducts of human-nature industries (i.e. fish processing waste, or bloodmeal from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine Triphosphate
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer. When consumed in metabolic processes, it converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. The human body recycles its own body weight equivalent in ATP each day. It is also a precursor to DNA and RNA, and is used as a coenzyme. From the perspective of biochemistry, ATP is classified as a nucleoside triphosphate, which indicates that it consists of three components: a nitrogenous base ( adenine), the sugar ribose, and the triphosphate. Structure ATP consists of an adenine attached by the 9-nitrogen atom to the 1′ carbon atom of a sugar ( ribose), which in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |