|
Phlorofucofuroeckol A
Phlorofucofuroeckol A is a phlorotannin isolated from brown algae species such as ''Eisenia bicyclis'' (an edible seaweed called ''arame'' in Japan), ''Ecklonia cava'', ''Ecklonia kurome'' or ''Ecklonia stolonifera''. The molecule possesses both the dibenzo-1,4-dioxin and dibenzofuran Dibenzofuran is a heterocyclic organic compound with the chemical structure shown at right. It is an aromatic compound that has two benzene rings fused to a central furan ring. All the numbered carbon atoms have a hydrogen atom bonded to each of th ... elements. References Benzofuran ethers at the benzene ring Dibenzofurans Phlorotannins {{aromatic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phlorotannin
Phlorotannins are a type of tannins found in brown algae such as kelps and rockweeds or sargassacean species, and in a lower amount also in some red algae. Contrary to hydrolysable or condensed tannins, these compounds are oligomers of phloroglucinol (polyphloroglucinols). As they are called tannins, they have the ability to precipitate proteins. It has been noticed that some phlorotannins have the ability to oxidize and form covalent bonds with some proteins. In contrast, under similar experimental conditions three types of terrestrial tannins (procyanidins, profisetinidins, and gallotannins) apparently did not form covalent complexes with proteins. These phenolic compounds are integral structural components of cell walls in brown algae, but they also seem to play many other secondary ecological roles such as protection from UV radiation and defense against grazing. Biosynthesis and localization Most of the phlorotannins' biosynthesis is still unknown, but it appears they ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eisenia Bicyclis
, sea oak is a species of kelp, of the brown algae, best known for its use in Japanese cuisine. Description ''Eisenia bicyclis'' is indigenous to temperate Pacific Ocean waters centered near Japan, although it is deliberately cultured elsewhere, including South Korea. Arame , Food to Good Health, 2010 retrieved 8 February 2013 It grows and reproduces seasonally. Two flattened oval fronds rise from a stiff woody stipe which can be up to about tall. The fronds are shed and new ones formed annually. The plant appears both branched and feathered. It may be harvested by divers manually or mechanically, and the dried form is available year-round. Cuisine It is one of many species of |
Ecklonia Cava
''Ecklonia cava'' (or paddle weed, , ''noro-kajime''), is an edible marine brown alga species found in the ocean off Japan and Korea. It is used as an herbal remedy in the form of an extract called ''Seanol'', a polyphenolic extract, and ''Ventol'', a phlorotannin-rich natural agent. Phlorotannins, such as fucodiphlorethol G, 7-phloro eckol, 6,6'-bieckol, eckol, 8,8'-bieckol, 8,4"'-dieckol and phlorofucofuroeckol A can be isolated from ''Ecklonia cava''. Other components are common sterol derivatives ( fucosterol, ergosterol and cholesterol). It is also identified as a viable colloid source for use in the biotech industry. Nomenclature ''Ecklonia cava'' answers to the English common name "paddle weed"; it is also referred by the common names "''kajime''" or "''noro-kajime''" of Japanese origin. In fact, the standard common name for ''E. cava'' in modern-day Japanese is , to be distinguished from the wrinkled-leaved ''Eisenia bicyclis'' ( ''Ecklonia bicyclis'') known ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ecklonia Kurome
''Ecklonia kurome'' ( ja, 黑布 (kurome), zh, 鹅掌菜) is a brown alga species in the genus ''Ecklonia'' found in the Sea of Japan. The phlorotannins eckol, phlorofucofuroeckol A and 8,8'-bieckol can be found in ''Ecklonia kurome''. An oligosaccharide extract from ''Ecklonia kurome'' called is approved in China for the treatment of Alzheimer's disease, but the evidence is highly dubious. See also * Kombu ''Konbu'' (from ja, 昆布, konbu or kombu) is edible kelp mostly from the family Laminariaceae and is widely eaten in East Asia. It may also be referred to as ''dasima'' ( ko, 다시마) or ''haidai'' (). Kelp features in the diets of many ... References External links algaebase.org kurome Plants described in 1927 {{Phaeophyceae-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ecklonia Stolonifera
''Ecklonia stolonifera'' (Japanese: ツルアラメ, turuarame) is a brown alga species in the genus ''Ecklonia'' found in the Sea of Japan. It is an edible species traditionally eaten in Japan. Chemistry Phlorotannins ''Ecklonia stolonifera'' contains the phlorotannins phlorofucofuroeckol A, eckol, dieckol, dioxinodehydroeckol (eckstolonol), 2-phloroeckol, phlorofucofuroeckol B, 6,6'-bieckol, triphlorethol-A, phloroglucinol and 7-phloroeckol. Those phlorotannins are responsible for the potent pharmacological effects associated with this seaweed. These molecules show a hepatoprotective activity. Oxylipins The oxylipins ecklonialactones A, B, C, D, E and F and fucosterol can also be isolated from the species. References External links algaebase.org {{Taxonbar, from=Q5332926 stolonifera Stolonifera is a suborder of soft corals in the order Alcyonacea. Members of this taxon are characterised by having separate polyps budding off an encrusting horiz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dibenzo-1,4-dioxin
Dibenzo-1,4-dioxin, also dibenzodioxin or dibenzo-''p''-dioxin (dibenzo-''para''-dioxin), is a polycyclic heterocyclic organic compound in which two benzene rings are connected by a 1,4-dioxin ring. Its molecular formula is C12H8O2. The two oxygen atoms occupy opposite ( ''para''-) positions in the six-membered dioxin ring. Dibenzodioxin is the carbon skeleton of the poisonous polychlorinated dibenzodioxins (PCDDs), often called dioxins. The most harmful PCDD is 2,3,7,8-tetrachlorodibenzodioxin (TCDD). Dioxins and dioxin-like compounds is a category of pollutants that includes PCDDs and other compounds that have similar structure, toxicity, and persistence. Dibenzodioxin is also the skeleton of the polybrominated dibenzodioxins. Isomer The general name dibenzodioxin usually refers to dibenzo-''p''-dioxin. The isomeric compound dibenzo-''o''-dioxin (dibenzo-''ortho''-dioxin) or dibenzo-1,2-dioxin, like the unstable 1,2-dioxin, has two adjacent oxygen atoms ( ''ortho''-). No ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dibenzofuran
Dibenzofuran is a heterocyclic organic compound with the chemical structure shown at right. It is an aromatic compound that has two benzene rings fused to a central furan ring. All the numbered carbon atoms have a hydrogen atom bonded to each of them. It is a volatile white solid that is soluble in nonpolar organic solvents. It is obtained from coal tar, where it exists as a 1% component.Gerd Collin and Hartmut Höke "Benzofurans" in Ullmann's Encyclopedia of Industrial Chemistry, 2007, Wiley-VCH, Weinheim. Reactions Dibenzofuran is thermally robust with a convenient liquid range. These properties, together with its low toxicity, are exploited by the use of DBF as a heat transfer agent. It undergoes electrophilic reactions, such as halogenation and Friedel-Crafts reactions. Reaction of DBF with butyl lithium results in dilithiation.Ulrich Iserloh, Yoji Oderaotoshi, Shuji Kanemasa, and Dennis P. Curran "Synthesis of (R,R)-4,6-Dibenzofurandiyl-2,2'-Bis (4-Phenyloxazoline) (DBFO ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
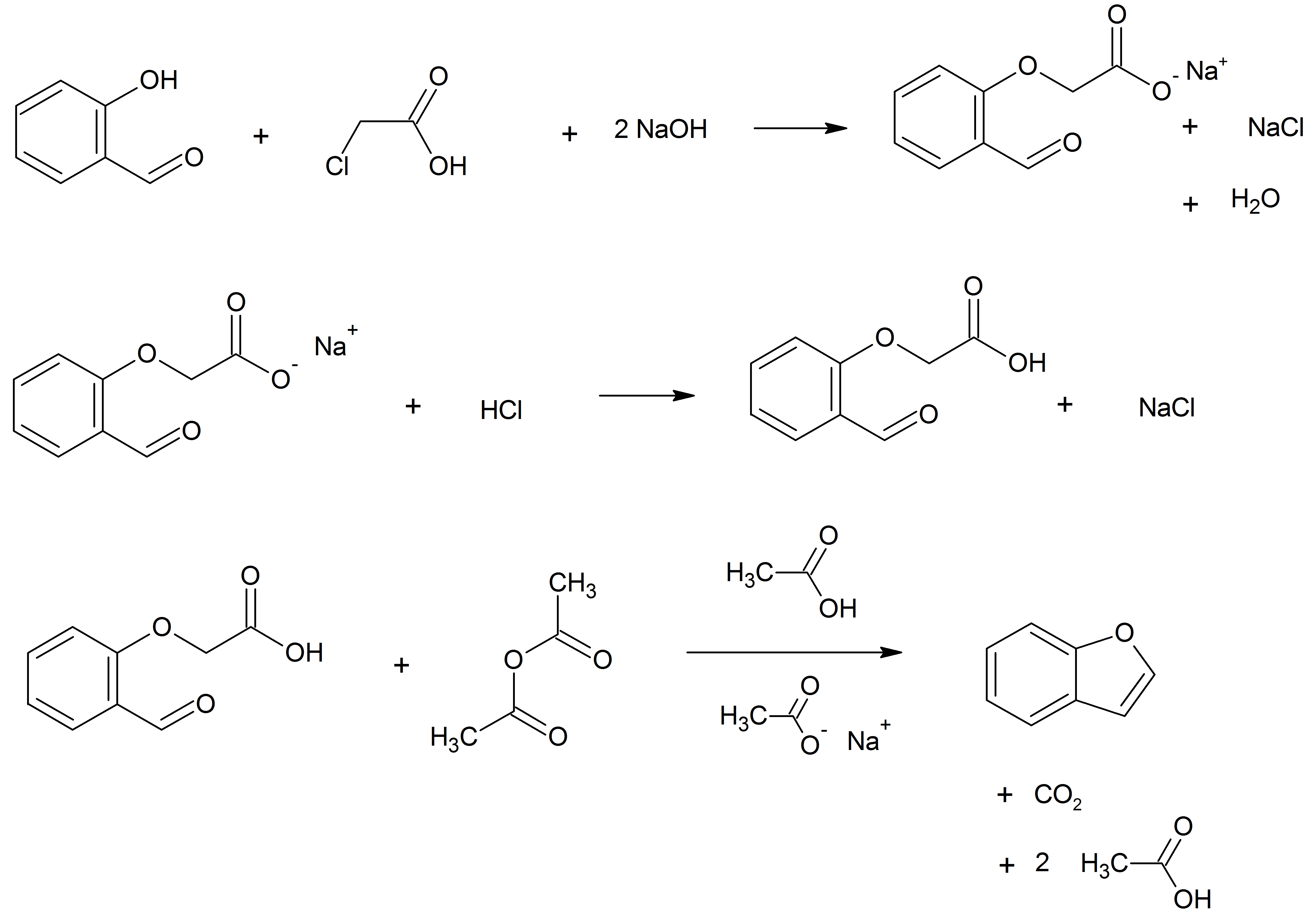
Benzofuran Ethers At The Benzene Ring
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. *Perkin rearrangement, where a coumarin is reacted with a hydroxide: : * Diels–Alder reaction of nitro vinyl furans with various dienophiles: : Diels–Alder reaction yielding a substituted benzofuran, 450px * Cycloisomerization of alkyne ortho-substituted phenols: : Benzofurans via Cycloisomerization, 400px Related co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dibenzofurans
Dibenzofuran is a heterocyclic organic compound with the chemical structure shown at right. It is an aromatic compound that has two benzene rings fused to a central furan ring. All the numbered carbon atoms have a hydrogen atom bonded to each of them. It is a volatile white solid that is soluble in nonpolar organic solvents. It is obtained from coal tar, where it exists as a 1% component.Gerd Collin and Hartmut Höke "Benzofurans" in Ullmann's Encyclopedia of Industrial Chemistry, 2007, Wiley-VCH, Weinheim. Reactions Dibenzofuran is thermally robust with a convenient liquid range. These properties, together with its low toxicity, are exploited by the use of DBF as a heat transfer agent. It undergoes electrophilic reactions, such as halogenation and Friedel-Crafts reactions. Reaction of DBF with butyl lithium results in dilithiation.Ulrich Iserloh, Yoji Oderaotoshi, Shuji Kanemasa, and Dennis P. Curran "Synthesis of (R,R)-4,6-Dibenzofurandiyl-2,2'-Bis (4-Phenyloxazoline) (DBFO ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |