|
Phenyl Azide
Phenyl azide is an organic compound with the formula C6H5N3. It is one of the prototypical organic azides. It is a pale yellow oily liquid with a pungent odor. The structure consists of a linear organic azide, azide substituent bound to a phenyl group. The C−N=N angle is approximately 120°. Preparation Phenyl azide is prepared by the Diazonium compound, diazotization of phenylhydrazine with nitrous acid: :C6H5NHNH2 + HNO2 → C6H5N3 + 2 H2O Aryl iodides bearing electron-withdrawing substituents undergo metathesis with sodium azide in the presence of Cu(I), sodium ascorbate, and 1,2-Dimethylethylenediamine, N,N'-dimethylethane-1,2-diamine (DMEDA): :RC6H4I + NaN3 → RC6H4N3 + NaI It can also be prepared by condensation of benzenediazonium salt with toluenesulfonamide, followed by hydrolysis. Chemical reactions Phenyl azide cycloadds to alkenes and especially Azide alkyne Huisgen cycloaddition, alkynes, particularly those bearing electronegative substituents. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society (professional association) in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 34,000 in the UK and a further 8,000 abroad. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azide Alkyne Huisgen Cycloaddition
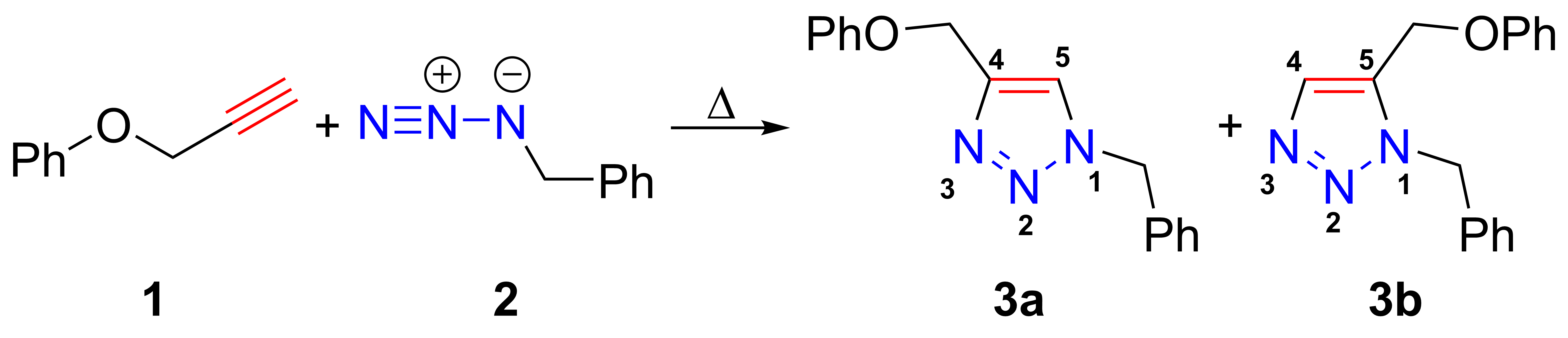
The azide-alkyne Huisgen cycloaddition is a 1,3-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 1,2,3-triazole. Rolf Huisgen was the first to understand the scope of this organic reaction. American chemist Karl Barry Sharpless has referred to this cycloaddition as "the cream of the crop" of click chemistry and "the premier example of a click reaction". In the reaction above azide 2 reacts neatly with alkyne 1 to afford the product triazole as a mixture of 1,4-adduct (3a) and 1,5-adduct (3b) at 98 °C in 18 hours. The standard 1,3-cycloaddition between an azide 1,3-dipole and an alkene as dipolarophile has largely been ignored due to lack of reactivity as a result of electron-poor olefins and elimination side reactions. Some success has been found with non-metal-catalyzed cycloadditions, such as the reactions using dipolarophiles that are electron-poor olefins or alkynes. Although azides are not the most reactive 1,3-dipole available ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenyl Compounds
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. Phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring. Nomenclature Usually, a "phenyl group" is synonymous with C6H5− and is represented by the symbol Ph or, archaically, Φ. Benzene is sometimes denoted as PhH. Phenyl groups are generally attached to other atoms or groups. For example, triphenylmethane (Ph3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Footnotes
A note is a string of text placed at the bottom of a page in a book or document or at the end of a chapter, volume, or the whole text. The note can provide an author's comments on the main text or citations of a reference work in support of the text. Footnotes are notes at the foot of the page while endnotes are collected under a separate heading at the end of a chapter, volume, or entire work. Unlike footnotes, endnotes have the advantage of not affecting the layout of the main text, but may cause inconvenience to readers who have to move back and forth between the main text and the endnotes. In some editions of the Bible, notes are placed in a narrow column in the middle of each page between two columns of biblical text. Numbering and symbols In English, a footnote or endnote is normally flagged by a superscripted number immediately following that portion of the text the note references, each such footnote being numbered sequentially. Occasionally, a number between brack ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and experiments reported in an article must be successfully repeated in the laboratory of a member of the editorial board as a check for reproducibility prior to publication. The journal is published by Organic Syntheses, Inc., a non-profit corporation. An annual print version is published by John Wiley & Sons on behalf of Organic Syntheses, Inc. History Prior to World War I, work on synthetic organic chemistry in the United States had been quite limited, and most of the reagents used in laboratories had to be imported from Europe. When export stoppages and trade embargoes cut off this source, Clarence Derick, a professor of chemistry at University of Illinois at Urbana-Champaign, began an effort to synthesize these needed chemicals in industr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blast Shield
Blast or The Blast may refer to: *Explosion, a rapid increase in volume and release of energy in an extreme manner *Detonation, an exothermic front accelerating through a medium that eventually drives a shock front Film * ''Blast'' (1997 film), starring Andrew Divoff * ''Blast'' (2000 film), starring Liesel Matthews * ''Blast'' (2004 film), an action comedy film * ''Blast!'' (1972 film) or ''The Final Comedown'', an American drama * ''BLAST!'' (2008 film), a documentary about the BLAST telescope * '' A Blast'', a 2014 film directed by Syllas Tzoumerkas Magazines * ''Blast'' (magazine), a 1914–15 literary magazine of the Vorticist movement * ''Blast'' (U.S. magazine), a 1933–34 American short-story magazine * ''The Blast'' (magazine), a 1916–17 American anarchist periodical Music * Blast (American band), a hardcore punk band * Blast (Russian band), an indie band * ''Blast'' (album), by Holly Johnson, 1989 * ''The Blast'' (album), by Yuvan Shankar Raja, 1999 * "Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Decomposition
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is required to break chemical bonds in the compound undergoing decomposition. If decomposition is sufficiently exothermic, a positive feedback loop is created producing thermal runaway and possibly an explosion or other chemical reaction. Decomposition temperature definition A simple substance (like water) may exist in equilibrium with its thermal decomposition products, effectively halting the decomposition. The equilibrium fraction of decomposed molecules increases with the temperature. Examples * Calcium carbonate (limestone or chalk) decomposes into calcium oxide and carbon dioxide when heated. The chemical reaction is as follows: ::CaCO3 → CaO + CO2 :The reaction is used to make Calcium oxide, quick lime, which is an industrially impor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylphosphine Phenylimide
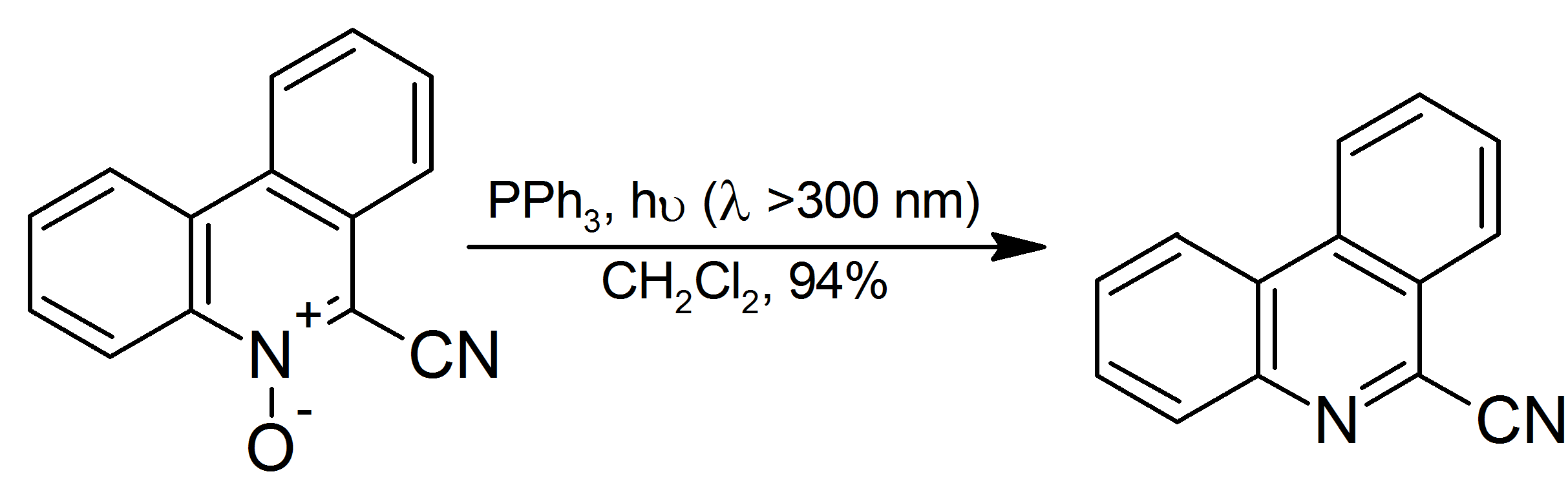
Triphenylphosphine phenylimide is the organophosphorus compound with the formula Ph3P=NPh ( Ph = C6H5). It is a white solid that is soluble in organic solvents. The compound is a prototype of a large class of Staudinger reagents, resulting from the Staudinger reaction. The phosphine imides were first prepared in the laboratory of Nobelist Hermann Staudinger. His synthesis involved the direct reaction of triphenylphosphine with phenylazide. :Ph3P + N3Ph → Ph3P=NPh + N2 X-ray crystallography X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ... establishes that the P-N-C angle is bent (130.4°) and the P-N distance is 160 pm.{{cite journal, title=Die Kristallstrukturen von Ph3PNPh, h3PN(H)PhAuI2], und von 2,3-Bis(triphenylphosphoranimino)maleinsäure-N-methylimid (The Cr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether. Preparation and structure Triphenylphosphine can be prepared in the laboratory by treatment of phosphorus trichloride with phenylmagnesium bromide or phenyllithium. The industrial synthesis involves the reaction between phosphorus trichloride, chlorobenzene, and sodium: :PCl3 + 3 PhCl + 6 Na → PPh3 + 6 NaCl Triphenylphosphine crystallizes in triclinic and monoclinic modification. In both cases, the molecule adopts a pyramidal structure with propeller-like arrangement of the three phenyl groups. Principal reactions with chalcogens, halogens, and acids Oxidation Triphenylphosphine undergoes slow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triazole
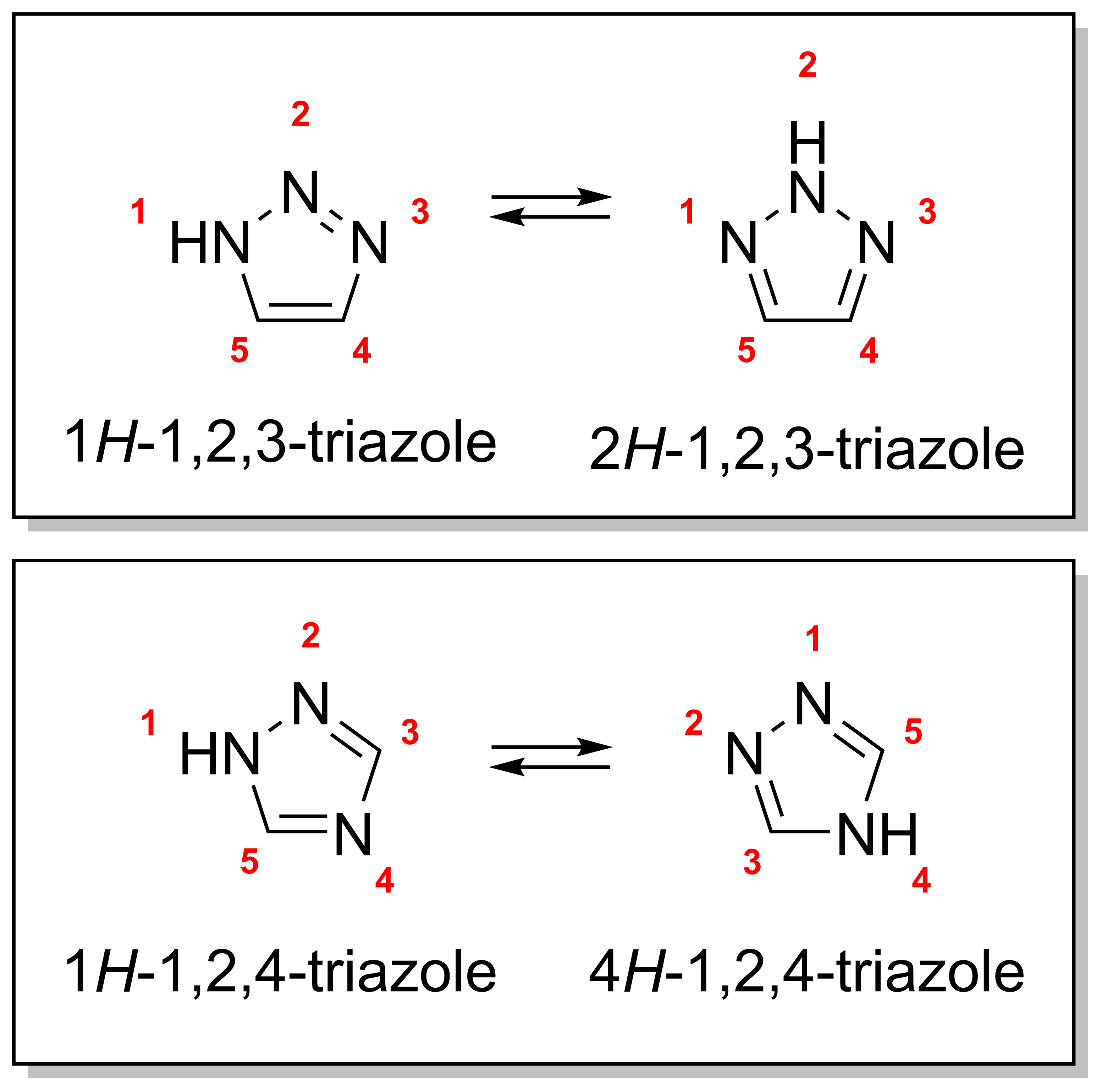
A triazole is a heterocyclic compound featuring a five-membered ring of two carbon atoms and three nitrogen atoms with molecular formula C2H3N3. Triazoles exhibit substantial isomerism, depending on the positioning of the nitrogen atoms within the ring. Many triazoles are versatile, biologically active compounds commonly used as fungicides and plant retardants. However, triazoles are also useful in bioorthogonal chemistry, because the large number of nitrogen atoms causes triazoles to react similar to azides. Lastly, the many free lone pairs in triazoles make them useful as coordination compounds, although not typically as haptic ligands. Isomerism There are four triazole isomers, which are conventionally divided into two pairs of tautomers. In the 1,2,3-triazoles, the three nitrogen atoms are adjacent; in the 1,2,4-triazoles, an interstitial carbon separates out one nitrogen atom. Each category has two tautomers that differ by which nitrogen has a hydrogen bonded ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas. Preparation In the laboratory, phenylacetylene can be prepared by elimination of hydrogen bromide from styrene dibromide using sodium amide in ammonia: : It can also be prepared by the elimination of hydrogen bromide from bromostyrene using molten potassium hydroxide. Reactions Phenylacetylene is a prototypical terminal acetylene, undergoing many reactions expected of that functional group. It undergoes semihydrogenated over Lindlar catalyst to give styrene. In the presence of base and copper(II) salts, it undergoes oxidative coupling to give diphenylbutadiyne. In the presence of metal catalysts, it undergoes oligomerization, trimerization, and even polymerization. : In the presence of gold or mercury reagents, phenylacetylene hydrates to give acet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Click Chemistry
In chemical synthesis, click chemistry is a class of biocompatible small molecule reactions commonly used in bioconjugation, allowing the joining of substrates of choice with specific biomolecules. Click chemistry is not a single specific reaction, but describes a way of generating products that follow examples in nature, which also generates substances by joining small modular units. In many applications, click reactions join a biomolecule and a reporter molecule. Click chemistry is not limited to biological conditions: the concept of a "click" reaction has been used in chemoproteomic, pharmacological, and various biomimetic applications. However, they have been made notably useful in the detection, localization and qualification of biomolecules. Click reactions occur in one pot, are not disturbed by water, generate minimal and inoffensive byproducts, and are "spring-loaded"—characterized by a high thermodynamic driving force that drives it quickly and irreversibly to high yi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |