Triazole on:
[Wikipedia]
[Google]
[Amazon]
A triazole is a heterocyclic compound featuring a five-membered ring of two carbon atoms and three nitrogen atoms with
Synthesis of 1,2,3-triazoles (overview of recent methods)
Synthesis of 1,2,4-triazoles (overview of recent methods)
molecular formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
C2H3N3. Triazoles exhibit substantial isomerism, depending on the positioning of the nitrogen atoms within the ring.
Many triazoles are versatile, biologically active compounds commonly used as fungicides and plant retardants. However, triazoles are also useful in bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept ...
, because the large number of nitrogen atoms causes triazoles to react similar to azides
In chemistry, azide is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant applicat ...
. Lastly, the many free lone pairs in triazoles make them useful as coordination compounds, although not typically as haptic ligands.
Isomerism
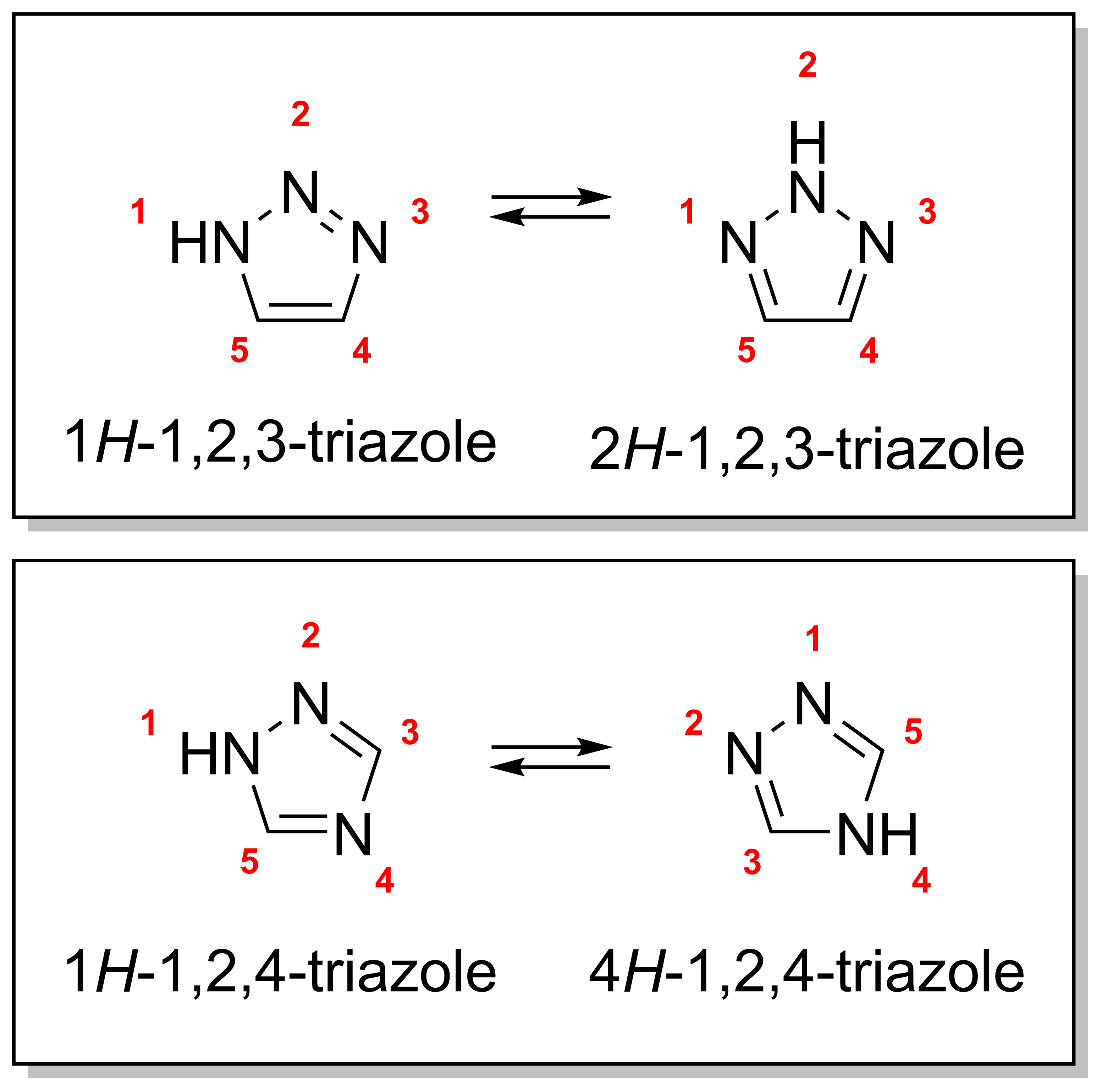
There are four triazole isomers, which are conventionally divided into two pairs oftautomers
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hyd ...
. In the 1,2,3-triazoles, the three nitrogen atoms are adjacent; in the 1,2,4-triazole
1,2,4-Triazole (as ligand in coordination compounds, Htrz abbreviation is sometimes used) is one of a pair of isomeric chemical compounds with molecular formula CHN, called triazoles, which have a five-membered ring of two carbon atoms and three n ...
s, an interstitial carbon separates out one nitrogen atom. Each category has two tautomer
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hyd ...
s that differ by which nitrogen has a hydrogen bonded to it.

Preparation
There are several methods to prepare triazoles.1,2,3-Triazoles
1,2,3-Triazoles are usually prepared following (3+2)cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity". T ...
protocols. A common technique for unsubstituted triazoles is the Huisgen azide-alkyne 1,3-dipolar cycloaddition: a azide and an alkyne react at high temperature to form a ring. However, the Huisgen strategy produces a mixture of isomers (typically 1,4- and 1,5-disubstituted) when used to produce substituted triazoles.
In order to selectively prepare a desired isomer, metal catalysts are employed. In the copper-catalysed azide-alkyne cycloaddition (CuAAC), copper(I) salts select for the formation of 1,4-disubstituted 1,2,3-triazoles. One such catalyst is CuBr(PPh3)3, which is relatively stable towards oxidation even at elevated temperatures and can produce triazoles with a broad range of substituents either in solvent or under ''neat'' reaction conditions.
Conversely, ruthenium catalysts (RuAAC) select for 1,5-disubstituted 1,2,3-triazoles.
1,2,4-Triazoles
Most techniques for producing 1,2,4-triazoles use the free energy of water, either by dehydrating a mixture ofamides
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is ...
and hydrazides (the Pellizzari reaction) or imides
In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant to hydrolysis. In terms of commercial applications, ...
and alkyl hydrazines (the Einhorn-Brunner reaction). Of those two, only the Einhorn-Brunner reaction is regioselective. Recent research has focused on grinding and microwave irradiation as greener substitutes.
Applications
Triazoles are compounds with a vast spectrum of applications, varying from materials (polymers), agricultural chemicals, pharmaceuticals, photoactive chemicals and dyes.Benzotriazole
Benzotriazole (BTA) is a heterocyclic compound with the chemical formula C6H5N3. Its five-membered ring contains three consecutive nitrogen atoms. This bicyclic compound may be viewed as fused rings of the aromatic compounds benzene and triazole. ...
is used in chemical photography as a restrainer and fog suppressant.
Cyclohexylethyltriazol was briefly used as an alternative to Cardiazol (Metrazol) in convulsive shock therapy treatment of mental illnesses during the 1940s.
Importance in agriculture
Many triazoles have antifungal effects: the triazoleantifungal drug
An antifungal medication, also known as an antimycotic medication, is a pharmaceutical fungicide or fungistatic used to treat and prevent mycosis such as athlete's foot, ringworm, candidiasis (thrush), serious systemic infections such as Cryptoc ...
s include fluconazole
Fluconazole is an antifungal medication used for a number of fungal infections. This includes candidiasis, blastomycosis, coccidiodomycosis, cryptococcosis, histoplasmosis, dermatophytosis, and pityriasis versicolor. It is also used to pr ...
, isavuconazole
Isavuconazonium sulfate, sold under the brand name Cresemba, is a systemic antifungal medication of the triazole class which is used to treat invasive aspergillosis and mucormycosis.
The most common side effects include abnormal liver tests, ...
, itraconazole
Itraconazole, sometimes abbreviated ITZ, is an antifungal medication used to treat a number of fungal infections. This includes aspergillosis, blastomycosis, coccidioidomycosis, histoplasmosis, and paracoccidioidomycosis. It may be given by mo ...
, voriconazole
Voriconazole, sold under the brand name Vfend among others, is an antifungal medication used to treat a number of fungal infections. This includes aspergillosis, candidiasis, coccidioidomycosis, histoplasmosis, penicilliosis, and infections by ...
, pramiconazole, ravuconazole, and posaconazole
Posaconazole, sold under the brand name Noxafil among others, is a triazole antifungal medication.
It was approved for medical use in the United States in September 2006, and is available as a generic medication.
Medical uses
Posaconazole is u ...
and triazole plant-protection fungicides include epoxiconazole
Epoxiconazole is a fungicide active ingredient from the class of azoles developed to protect crops. In particular, the substance inhibits the metabolism of fungi cells infesting useful plants, and thereby prevents the growth of the mycelia (funga ...
, , myclobutanil
Myclobutanil is a triazole chemical used as a fungicide. It is a steroid demethylation inhibitor, specifically inhibiting ergosterol biosynthesis. Ergosterol is a critical component of fungal cell membranes.
Stereoisomerism
Safety
The Safet ...
, propiconazole
Propiconazole is a triazole fungicide, also known as a DMI, or demethylation inhibiting fungicide due to its binding with and inhibiting the 14-alpha demethylase enzyme from demethylating a precursor to ergosterol. Without this demethylation ste ...
, prothioconazole, metconazole, cyproconazole
Cyproconazole is an agricultural fungicide of the class of azoles, used on cereal crops, coffee, sugar beet, fruit trees and grapes, on sod farms and golf courses and on wood as a preservative. It was introduced to the market by then Sandoz in 1 ...
, tebuconazole
Tebuconazole is a triazole fungicide used agriculturally to treat plant pathogenic fungi.
Environmental Hazards
Though the U.S. Food and Drug Administration considers this fungicide to be safe for humans, it may still pose a risk. It is listed ...
, flusilazole
Flusilazole (DPX-H6573) is an organosilicon fungicide invented by DuPont, which is used to control fungal infections on a variety of fruit and vegetable crops. It is moderately toxic to animal
Animals are multicellular, eukaryotic organ ...
and paclobutrazol
Paclobutrazol (PBZ) is the ISO common name for an organic compound that is used as a plant growth retardant and triazole fungicide. It is a known antagonist of the plant hormone gibberellin, acting by inhibiting gibberellin biosynthesis, reduc ...
.
Due to spreading resistance of plant pathogens towards fungicides of the strobilurin class, control of fungi such as '' Septoria tritici'' or '' Gibberella zeae'' relies heavily on triazoles. Food, like store bought potatoes, contain retardants such as triazole or tetcyclacis.
In addition, paclobutrazol
Paclobutrazol (PBZ) is the ISO common name for an organic compound that is used as a plant growth retardant and triazole fungicide. It is a known antagonist of the plant hormone gibberellin, acting by inhibiting gibberellin biosynthesis, reduc ...
, uniconazole, , and triadimefon are used as plant growth retardants. Brassinazole inhibits brassinosteroid
Brassinosteroids (BRs or less commonly BS) are a class of polyhydroxysteroids that have been recognized as a sixth class of
plant hormones and may have utility as an anticancer drug for endocrine-responsive cancers to induce apoptosis and inhibit ...
biosynthesis.
Importance in chemical synthesis
The azide alkyne Huisgen cycloaddition is a mild and selective reaction that gives 1,2,3-triazoles as products. The reaction has been widely used inbioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept ...
and in organic synthesis. Triazoles are relatively stable functional groups and triazole linkages can be used in a variety of applications, e.g. replacing the phosphate backbone of DNA.
Related heterocycles
*Imidazole
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-a ...
, an analog with two nonadjacent nitrogen atoms
* Pyrazole
Pyrazole is an organic compound with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazole is a weak base, with p''K''b 11.5 ( ...
, an analog with two adjacent nitrogen atoms
* Tetrazole
Tetrazoles are a class of synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen atoms and one carbon atom. The name tetrazole also refers to the parent compound with formula CH2N4, of which three isomers can be fo ...
, an analog with four nitrogen atoms
* Triazolium salts, substituted analogues that can be used as NHC precursors
External links
Synthesis of 1,2,3-triazoles (overview of recent methods)
Synthesis of 1,2,4-triazoles (overview of recent methods)
References
{{Authority control Chemical compounds Simple aromatic rings Triazoles