|
Pharmaceutical Microbiology
Pharmaceutical microbiology is an applied branch of microbiology. It involves the study of microorganisms associated with the manufacture of pharmaceuticals e.g. minimizing the number of microorganisms in a process environment, excluding microorganisms and microbial byproducts like exotoxin and endotoxin from water and other starting materials, and ensuring the finished pharmaceutical product is sterile. Other aspects of pharmaceutical microbiology include the research and development of antibiotics, anti-infective agents, the use of microorganisms to detect mutagenic and carcinogenic activity in prospective drugs, and the use of microorganisms in the manufacture of pharmaceutical products like insulin and human growth hormone. Drug safety Drug safety is a major focus of pharmaceutical microbiology. Pathogenic bacteria, fungi (yeasts and moulds) and toxins produced by microorganisms are all possible contaminants of medicines- although stringent, regulated processes are in plac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microbiology
Microbiology () is the scientific study of microorganisms, those being unicellular (single cell), multicellular (cell colony), or acellular (lacking cells). Microbiology encompasses numerous sub-disciplines including virology, bacteriology, protistology, mycology, immunology, and parasitology. Eukaryotic microorganisms possess membrane-bound organelles and include fungi and protists, whereas prokaryotic organisms—all of which are microorganisms—are conventionally classified as lacking membrane-bound organelles and include Bacteria and Archaea. Microbiologists traditionally relied on culture, staining, and microscopy. However, less than 1% of the microorganisms present in common environments can be cultured in isolation using current means. Microbiologists often rely on molecular biology tools such as DNA sequence based identification, for example the 16S rRNA gene sequence used for bacteria identification. Viruses have been variably classified as organisms, as they have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cough Medicine
Cold medicines are a group of medications taken individually or in combination as a treatment for the symptoms of the common cold and similar conditions of the upper respiratory tract. The term encompasses a broad array of drugs, including analgesics, antihistamines and decongestants, among many others. It also includes drugs which are marketed as cough suppressants or antitussives, but their effectiveness in reducing cough symptoms is unclear or minimal. While they have been used by 10% of American children in any given week, they are not recommended in Canada or the United States in children six years or younger because of lack of evidence showing effect and concerns of harm. In 2020, one version containing codeine and guaifenesin was the 377th most commonly prescribed medication in the United States with more than 400thousand prescriptions. Types There are a number of different cough and cold medications, which may be used for various coughing symptoms. The commercially ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microorganisms
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in older texts. The informal synonym ''microbe'' () comes from μικρός, mikrós, "small" and βίος, bíos, "life". is an organism of microscopic size, which may exist in its single-celled form or as a colony of cells. The possible existence of unseen microbial life was suspected from ancient times, such as in Jain scriptures from sixth century BC India. The scientific study of microorganisms began with their observation under the microscope in the 1670s by Anton van Leeuwenhoek. In the 1850s, Louis Pasteur found that microorganisms caused food spoilage, debunking the theory of spontaneous generation. In the 1880s, Robert Koch discovered that microorganisms caused the diseases tuberculosis, cholera, diphtheria, and anthrax. Because micr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specifications
A specification often refers to a set of documented requirements to be satisfied by a material, design, product, or service. A specification is often a type of technical standard. There are different types of technical or engineering specifications (specs), and the term is used differently in different technical contexts. They often refer to particular documents, and/or particular information within them. The word ''specification'' is broadly defined as "to state explicitly or in detail" or "to be specific". A requirement specification is a documented requirement, or set of documented requirements, to be satisfied by a given material, design, product, service, etc. It is a common early part of engineering design and product development processes in many fields. A functional specification is a kind of requirement specification, and may show functional block diagrams. A design or product specification describes the features of the ''solutions'' for the Requirement Specification, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Pharmacopeia
The ''European Pharmacopoeia'' (''Pharmacopoeia Europaea'', ''Ph. Eur.'') is a major regional pharmacopoeia which provides common quality standards throughout the pharmaceutical industry in Europe to control the quality of medicines, and the substances used to manufacture them. It is a published collection of monographs which describe both the individual and general quality standards for ingredients, dosage forms, and methods of analysis for medicines. These standards apply to medicines for both human and veterinary use. Legal basis The ''European Pharmacopoeia'' has a legally binding character. It is used as an official reference to serve public health, and is part of the regulatory requirements for obtaining a Marketing Authorisation (MA) for a medicinal (human or veterinary) product. The quality standards of the European Pharmacopoeia apply throughout the entire life-cycle of a product, and become legally binding and mandatory on the same date in all thirty-nine (39) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
British Pharmacopoeia
The ''British Pharmacopoeia'' (''BP'') is the national pharmacopoeia of the United Kingdom. It is an annually published collection of quality standards for medicinal substances in the UK, which is used by individuals and organisations involved in pharmaceutical research, development, manufacture and testing. Pharmacopoeial standards are publicly available and legally enforceable standards of quality for medicinal products and their constituents. The ''British Pharmacopoeia'' is an important statutory component in the control of medicines, which complements and assists the licensing and inspection processes of the UK's Medicines and Healthcare products Regulatory Agency (MHRA). Together with the ''British National Formulary'' (BNF), the ''British Pharmacopoeia'' defines the UK's pharmaceutical standards. Pharmacopoeial standards are compliance requirements; that is, they provide the means for an independent judgement as to the overall quality of an article, and apply throu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Japanese Pharmacopeia
The is the official pharmacopoeia of Japan. It is published by the . The first edition was published on 25 June 1886, with revisions being issued from time to time. The current revision is number 18, issued electronically on 7 June 2021. An official English translation is in preparation (status: 06 Aug 2021). See also * ''The International Pharmacopoeia ''The International Pharmacopoeia'' (''Pharmacopoeia Internationalis'', ''Ph. Int.'') is a pharmacopoeia issued by the World Health Organization as a recommendation, with the aim to provide international quality specifications for pharmaceutical s ...'' References Pharmacopoeias Pharmacy in Japan {{japan-lit-stub ja:薬局方#日本薬局方 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
United States Pharmacopeia
The ''United States Pharmacopeia'' (''USP'') is a pharmacopeia (compendium of drug information) for the United States published annually by the United States Pharmacopeial Convention (usually also called the USP), a nonprofit organization that owns the trademark and also owns the copyright on the pharmacopeia itself. The ''USP'' is published in a combined volume with the ''National Formulary'' (a formulary) as the USP-NF. If a drug ingredient or drug product has an applicable ''USP'' quality standard (in the form of a ''USP-NF'' monograph), it must conform in order to use the designation "USP" or "NF". Drugs subject to ''USP'' standards include both human drugs ( prescription, over-the-counter, or otherwise) and animal drugs. ''USP-NF'' standards also have a role in US federal law; a drug or drug ingredient with a name recognized in ''USP-NF'' is considered adulterated if it does not satisfy compendial standards for strength, quality or purity. USP also sets standards for diet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmacopeia
A pharmacopoeia, pharmacopeia, or pharmacopoea (from the obsolete typography ''pharmacopœia'', meaning "drug-making"), in its modern technical sense, is a book containing directions for the identification of compound medicines, and published by the authority of a government or a medical or pharmaceutical society. Descriptions of preparations are called monographs. In a broader sense it is a reference work for pharmaceutical drug specifications. Etymology The term derives from grc, φαρμακοποιία ''pharmakopoiia'' "making of (healing) medicine, drug-making", a compound of φάρμακον ''pharmakon'' "healing medicine, drug, poison", the verb ποιεῖν ''poiein'' "to make" and the abstract noun suffix -ία ''-ia''. In early modern editions of Latin texts, the Greek diphthong οι (''oi'') is latinized to its Latin equivalent ''oe'' which is in turn written with the ligature ''œ'', giving the spelling ''pharmacopœia''; in modern UK English, ''œ'' is writ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
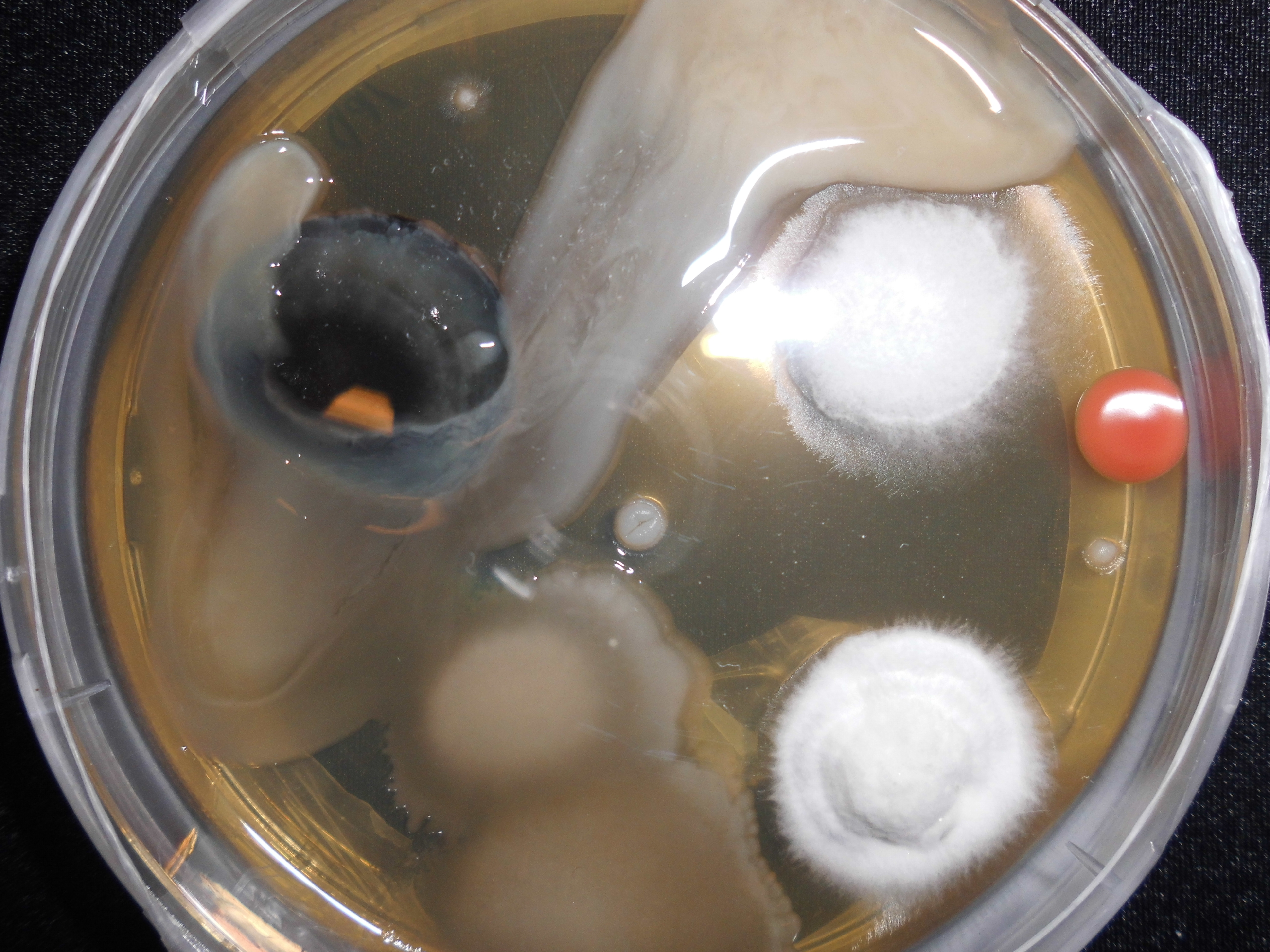
Candida Albicans
''Candida albicans'' is an opportunistic pathogenic yeast that is a common member of the human gut flora. It can also survive outside the human body. It is detected in the gastrointestinal tract and mouth in 40–60% of healthy adults. It is usually a commensal organism, but it can become pathogenic in immunocompromised individuals under a variety of conditions. It is one of the few species of the genus '' Candida'' that causes the human infection candidiasis, which results from an overgrowth of the fungus. Candidiasis is, for example, often observed in HIV-infected patients. ''C. albicans'' is the most common fungal species isolated from biofilms either formed on (permanent) implanted medical devices or on human tissue. ''C. albicans'', ''C. tropicalis'', ''C. parapsilosis'', and ''C. glabrata'' are together responsible for 50–90% of all cases of candidiasis in humans. A mortality rate of 40% has been reported for patients with systemic candidiasis due to ''C. albicans''. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Escherichia Coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escherichia'' that is commonly found in the lower intestine of warm-blooded organisms. Most ''E. coli'' strains are harmless, but some serotypes ( EPEC, ETEC etc.) can cause serious food poisoning in their hosts, and are occasionally responsible for food contamination incidents that prompt product recalls. Most strains do not cause disease in humans and are part of the normal microbiota of the gut; such strains are harmless or even beneficial to humans (although these strains tend to be less studied than the pathogenic ones). For example, some strains of ''E. coli'' benefit their hosts by producing vitamin K2 or by preventing the colonization of the intestine by pathogenic bacteria. These mutually beneficial relationships between ''E. col ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Contamination
Contamination is the presence of a constituent, impurity, or some other undesirable element that spoils, corrupts, infects, makes unfit, or makes inferior a material, physical body, natural environment, workplace, etc. Types of contamination Within the sciences, the word "contamination" can take on a variety of subtle differences in meaning, whether the contaminant is a solid or a liquid, as well as the variance of environment the contaminant is found to be in. A contaminant may even be more abstract, as in the case of an unwanted energy source that may interfere with a process. The following represent examples of different types of contamination based on these and other variances. Chemical contamination In chemistry, the term "contamination" usually describes a single constituent, but in specialized fields the term can also mean chemical mixtures, even up to the level of cellular materials. All chemicals contain some level of impurity. Contamination may be recognized or not a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |