|
Penciclovir
Penciclovir is a guanosine analogue antiviral drug used for the treatment of various herpesvirus infections. It is a nucleoside analogue which exhibits low toxicity and good selectivity. Because penciclovir is absorbed poorly when given orally (by mouth) it is more often used as a topical treatment. It is the active ingredient in the cold sore medications Denavir ( NDC 0135-0315-52), Vectavir and Fenivir. Famciclovir is a prodrug of penciclovir with improved oral bioavailability. Penciclovir was approved for medical use in 1996. Medical use In herpes labialis, the duration of healing, pain and detectable virus is reduced by up to one day,Fass.se">Farmaceutiska Specialiteter i Sverige - the Swedish official drug catalog Vectavir. Retrieved on August 12, 2009. Translated from "Tiden för läkning, smärta och påvisbart virus förkortas med upp till ett dygn." compared with the total duration of 2–3 weeks of disease presentation. Mechanism of action Penciclovir is inactive in i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Famciclovir
Famciclovir is a guanosine analogue antiviral drug used for the treatment of various herpesvirus infections, most commonly for herpes zoster (shingles). It is a prodrug form of penciclovir with improved oral bioavailability. Famciclovir is marketed under the trade name Famvir (Novartis). Famciclovir was patented in 1983 and approved for medical use in 1994. In 2007, the United States Food and Drug Administration approved the first generic version of famciclovir. Generic tablets are manufactured by TEVA Pharmaceuticals and Mylan Pharmaceuticals. Medical uses Famciclovir is indicated for the treatment of herpes zoster (shingles), treatment of herpes simplex virus 2 (genital herpes), herpes labialis (cold sores) in immunocompetent patients and for the suppression of recurring episodes of herpes simplex virus 2. It is also indicated for treatment of recurrent episodes of herpes simplex in HIV patients. Adverse effects Side effects: mild to extreme stomach upset, headaches, mild fev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleoside Analogue
Nucleoside analogues are nucleosides which contain a nucleic acid analogue and a sugar. Nucleotide analogs are nucleotides which contain a nucleic acid analogue, a sugar, and a phosphate group with one to three phosphates. Nucleoside and nucleotide analogues can be used in therapeutic drugs, including a range of antiviral products used to prevent viral replication in infected cells. The most commonly used is acyclovir, although its inclusion in this category is uncertain, because it acts as a nucleoside but contains no actual sugar, as the sugar ring is replaced by an open-chain structure. Nucleotide and nucleoside analogues can also be found naturally. Examples include ddhCTP (3ʹ-deoxy-3′,4ʹdidehydro-CTP) produced by the human antiviral protein viperin and sinefungin (a S-Adenosyl methionine analogue) produced by some ''Streptomyces''. Function These agents can be used against hepatitis B virus, hepatitis C virus, herpes simplex, and HIV. Once they are phosphorylat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleoside Analogues
Nucleoside analogues are nucleosides which contain a nucleic acid analogue and a sugar. Nucleotide analogs are nucleotides which contain a nucleic acid analogue, a sugar, and a phosphate group with one to three phosphates. Nucleoside and nucleotide analogues can be used in therapeutic drugs, including a range of antiviral products used to prevent viral replication in infected cells. The most commonly used is acyclovir, although its inclusion in this category is uncertain, because it acts as a nucleoside but contains no actual sugar, as the sugar ring is replaced by an open-chain structure. Nucleotide and nucleoside analogues can also be found naturally. Examples include ddhCTP (3ʹ-deoxy-3′,4ʹdidehydro-CTP) produced by the human antiviral protein viperin and sinefungin (a S-Adenosyl methionine analogue) produced by some ''Streptomyces''. Function These agents can be used against hepatitis B virus, hepatitis C virus, herpes simplex, and HIV. Once they are phosphorylat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Herpes Labialis
Herpes labialis, commonly known as cold sores or fever blisters, is a type of infection by the herpes simplex virus that affects primarily the lip. Symptoms typically include a burning pain followed by small blisters or sores. The first attack may also be accompanied by fever, sore throat, and enlarged lymph nodes. The rash usually heals within ten days, but the virus remains dormant in the trigeminal ganglion. The virus may periodically reactivate to create another outbreak of sores in the mouth or lip. The cause is usually herpes simplex virus type 1 (HSV-1) and occasionally herpes simplex virus type 2 (HSV-2). The infection is typically spread between people by direct non-sexual contact. Attacks can be triggered by sunlight, fever, psychological stress, or a menstrual period. Direct contact with the genitals can result in genital herpes. Diagnosis is usually based on symptoms but can be confirmed with specific testing. Prevention includes avoiding kissing or using the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
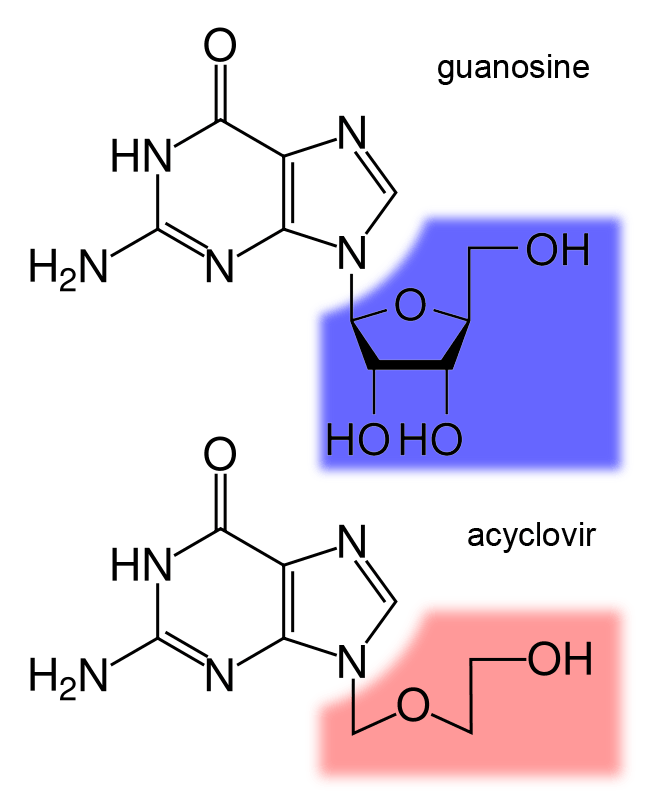
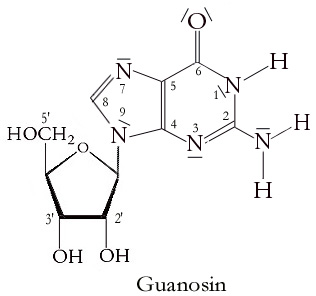
Guanosine
Guanosine (symbol G or Guo) is a purine nucleoside comprising guanine attached to a ribose (ribofuranose) ring via a β-N9-glycosidic bond. Guanosine can be phosphorylated to become guanosine monophosphate (GMP), cyclic guanosine monophosphate (cGMP), guanosine diphosphate (GDP), and guanosine triphosphate (GTP). These forms play important roles in various biochemical processes such as synthesis of nucleic acids and proteins, photosynthesis, muscle contraction, and intracellular signal transduction (cGMP). When guanine is attached by its N9 nitrogen to the C1 carbon of a deoxyribose ring it is known as deoxyguanosine. Physical and chemical properties Guanosine is a white, crystalline powder with no odor and mild saline taste. It is very soluble in acetic acid, slightly soluble in water, insoluble in ethanol, diethyl ether, benzene and chloroform. Functions Guanosine is required for an RNA splicing reaction in mRNA, when a "self-splicing" intron removes itself from the mRNA messag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DNA Polymerase
A DNA polymerase is a member of a family of enzymes that catalyze the synthesis of DNA molecules from nucleoside triphosphates, the molecular precursors of DNA. These enzymes are essential for DNA replication and usually work in groups to create two identical DNA duplexes from a single original DNA duplex. During this process, DNA polymerase "reads" the existing DNA strands to create two new strands that match the existing ones. These enzymes catalyze the chemical reaction : deoxynucleoside triphosphate + DNAn pyrophosphate + DNAn+1. DNA polymerase adds nucleotides to the three prime (3')-end of a DNA strand, one nucleotide at a time. Every time a cell divides, DNA polymerases are required to duplicate the cell's DNA, so that a copy of the original DNA molecule can be passed to each daughter cell. In this way, genetic information is passed down from generation to generation. Before replication can take place, an enzyme called helicase unwinds the DNA molecule from its tightl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aciclovir
Aciclovir (ACV), also known as acyclovir, is an antiviral medication. It is primarily used for the treatment of herpes simplex virus infections, chickenpox, and shingles. Other uses include prevention of cytomegalovirus infections following transplant and severe complications of Epstein–Barr virus infection. It can be taken by mouth, applied as a cream, or injected. Common side effects include nausea and diarrhea. Potentially serious side effects include kidney problems and low platelets. Greater care is recommended in those with poor liver or kidney function. It is generally considered safe for use in pregnancy with no harm having been observed. It appears to be safe during breastfeeding. Aciclovir is a nucleoside analogue that mimics guanosine. It works by decreasing the production of the virus's DNA. Aciclovir was patented in 1974, and approved for medical use in 1981. It is on the World Health Organization's List of Essential Medicines. It is available as a generic me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytotoxicity
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are an immune cell or some types of venom, e.g. from the puff adder (''Bitis arietans'') or brown recluse spider (''Loxosceles reclusa''). Cell physiology Treating cells with the cytotoxic compound can result in a variety of cell fates. The cells may undergo necrosis, in which they lose membrane integrity and die rapidly as a result of cell lysis. The cells can stop actively growing and dividing (a decrease in cell viability), or the cells can activate a genetic program of controlled cell death (apoptosis). Cells undergoing necrosis typically exhibit rapid swelling, lose membrane integrity, shut down metabolism, and release their contents into the environment. Cells that undergo rapid necrosis in vitro do not have sufficient time or energy to activate apoptotic machinery and will not express apoptotic markers. Apoptosis is characterized by well defined cytological and molecular events including a change i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selective Toxicity
*
*
{{disambig ...
Selective may refer to: * Selective school, a school that admits students on the basis of some sort of selection criteria ** Selective school (New South Wales) Selective strength: the human body transitions between being weak and strong. This ranges depending on the initial strength of the person. On some days for example you may be able to lift a heavy weight or open a difficult jar/bottle but in another day you won’t be able to do these things. See also * Selective breeding, the process of breeding for specific traits * Selection (other) * Selectivity (other) Selectivity may refer to: Psychology and behaviour * Choice, making a selection among options * Discrimination (information)#Biology and psychology, Discrimination, the ability to recognize differences * Socioemotional selectivity theory, in soci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thymidine Kinase
Thymidine kinase is an enzyme, a phosphotransferase (a kinase): 2'-deoxythymidine kinase, ATP-thymidine 5'-phosphotransferase, EC 2.7.1.21. It can be found in most living cells. It is present in two forms in mammalian cells, TK1 and TK2. Certain viruses also have genetic information for expression of viral thymidine kinases. Thymidine kinase catalyzes the reaction: :Thd + ATP → TMP + ADP where Thd is (deoxy)thymidine, ATP is adenosine triphosphate, TMP is (deoxy)thymidine monophosphate and ADP is adenosine diphosphate. Thymidine kinases have a key function in the synthesis of DNA and therefore in cell division, as they are part of the unique reaction chain to introduce thymidine into the DNA. Thymidine is present in the body fluids as a result of degradation of DNA from food and from dead cells. Thymidine kinase is required for the action of many antiviral drugs. It is used to select hybridoma cell lines in production of monoclonal antibodies. In clinical chemistry it is use ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinase
In biochemistry, a kinase () is an enzyme that catalyzes the transfer of phosphate groups from high-energy, phosphate-donating molecules to specific substrates. This process is known as phosphorylation, where the high-energy ATP molecule donates a phosphate group to the substrate molecule. This transesterification produces a phosphorylated substrate and ADP. Conversely, it is referred to as dephosphorylation when the phosphorylated substrate donates a phosphate group and ADP gains a phosphate group (producing a dephosphorylated substrate and the high energy molecule of ATP). These two processes, phosphorylation and dephosphorylation, occur four times during glycolysis. Kinases are part of the larger family of phosphotransferases. Kinases should not be confused with phosphorylases, which catalyze the addition of inorganic phosphate groups to an acceptor, nor with phosphatases, which remove phosphate groups (dephosphorylation). The phosphorylation state of a molecule, whet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid . The phosphate or orthophosphate ion is derived from phosphoric acid by the removal of three protons . Removal of one or two protons gives the dihydrogen phosphate ion and the hydrogen phosphate ion ion, respectively. These names are also used for salts of those anions, such as ammonium dihydrogen phosphate and trisodium phosphate. File:3-phosphoric-acid-3D-balls.png, Phosphoricacid File:2-dihydrogenphosphate-3D-balls.png, Dihydrogenphosphate File:1-hydrogenphosphate-3D-balls.png, Hydrogenphosphate File:0-phosphate-3D-balls.png, Phosphate In organic chemistry, phosphate or orthophosphate is an organophosphate, an ester of orthophosphoric acid of the form where one or more hydrogen atoms are replaced by organic groups. An example is trimethyl phosphate, . The term also refers to the triv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |