|
Parylene N Repeat Unit
Parylene is the common name of a polymer whose backbone consists of ''para''- benzenediyl rings –– connected by 1,2-ethanediyl bridges –––. It can be obtained by polymerization of ''para''-xylylene . The name is also used for several polymers with the same backbone, where some hydrogen atoms are replaced by other functional groups. Some of these variants are designated in commerce by letter-number codes such as "parylene C" and "parylene AF-4". Some of these names are registered trademarks in some countries. Coatings of parylene are often applied to electronic circuits and other equipment as electrical insulation, moisture barriers, or protection against corrosion and chemical attack. They are also used to reduce friction, and in medicine to prevent adverse reactions to implanted devices. These coatings are typically applied by chemical vapor deposition in an atmosphere of the monomer ''para''-xylylene. Parylene is considered a "green" polymer because it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parylene N Repeat Unit
Parylene is the common name of a polymer whose backbone consists of ''para''- benzenediyl rings –– connected by 1,2-ethanediyl bridges –––. It can be obtained by polymerization of ''para''-xylylene . The name is also used for several polymers with the same backbone, where some hydrogen atoms are replaced by other functional groups. Some of these variants are designated in commerce by letter-number codes such as "parylene C" and "parylene AF-4". Some of these names are registered trademarks in some countries. Coatings of parylene are often applied to electronic circuits and other equipment as electrical insulation, moisture barriers, or protection against corrosion and chemical attack. They are also used to reduce friction, and in medicine to prevent adverse reactions to implanted devices. These coatings are typically applied by chemical vapor deposition in an atmosphere of the monomer ''para''-xylylene. Parylene is considered a "green" polymer because it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Green Chemistry
Green chemistry, also called sustainable chemistry, is an area of chemistry and chemical engineering focused on the design of products and processes that minimize or eliminate the use and generation of hazardous substances. While environmental chemistry focuses on the effects of polluting chemicals on nature, green chemistry focuses on the environmental impact of chemistry, including lowering consumption of nonrenewable resources and technological approaches for preventing pollution. The overarching goals of green chemistry—namely, more resource-efficient and inherently safer design of molecules, materials, products, and processes—can be pursued in a wide range of contexts. History Green chemistry emerged from a variety of existing ideas and research efforts (such as atom economy and catalysis) in the period leading up to the 1990s, in the context of increasing attention to problems of chemical pollution and resource depletion. The development of green chemistry in Europe a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases. Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning 'flow' gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist Henri Moissan isolate elemental fluorine using low-temperature electrolysis, a process still employed for modern pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride gas and hydrochloric acid are important in technology and industry. Hydrochloric acid, the aqueous solution of hydrogen chloride, is also commonly given the formula HCl. Reactions Hydrogen chloride is a diatomic molecule, consisting of a hydrogen atom H and a chlorine atom Cl connected by a polar covalent bond. The chlorine atom is much more electronegative than the hydrogen atom, which makes this bond polar. Consequently, the molecule has a large dipole moment with a negative partial charge (δ−) at the chlorine atom and a positive partial charge (δ+) at the hydrogen atom. In part because of its high polarity, HCl is very soluble in water (and in other polar solvents). Upon contact, and HCl combine to form hydronium cations and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Printed Circuit Board
A printed circuit board (PCB; also printed wiring board or PWB) is a medium used in Electrical engineering, electrical and electronic engineering to connect electronic components to one another in a controlled manner. It takes the form of a Lamination, laminated sandwich structure of conductive and insulating layers: each of the conductive layers is designed with an artwork pattern of traces, planes and other features (similar to wires on a flat surface) Chemical milling, etched from one or more sheet layers of copper Lamination, laminated onto and/or between sheet layers of a Insulator (electricity), non-conductive substrate. Electrical components may be fixed to conductive pads on the outer layers in the shape designed to accept the component's terminals, generally by means of soldering, to both electrically connect and mechanically fasten them to it. Another manufacturing process adds Via (electronics), vias: plated-through holes that allow interconnections between layers. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RoHS
The Restriction of Hazardous Substances Directive 2002/95/EC (RoHS 1), short for Directive on the restriction of the use of certain hazardous substances in electrical and electronic equipment, was adopted in February 2003 by the European Union. The initiative was to prevent an overabundance of chemicals in electronics. Thus, as a result electronics were restricted. The RoHS 1 directive took effect on 1 July 2006, and is required to be enforced and became a law in each member state. This directive restricts (with exceptions) the use of ten hazardous materials in the manufacture of various types of electronic and electrical equipment. In addition to the exceptions, there are exclusions for products such as solar panels. It is closely linked with the Waste Electrical and Electronic Equipment Directive (WEEE) 2002/96/EC (now superseded) which sets collection, recycling and recovery targets for electrical goods and is part of a legislative initiative to solve the problem of h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Electronegativity#Pauling electronegativity, Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval Alchemy, alchemists, which commonly involved the heating of chloride Salt (chemistry), salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and hydrochloric acid (in the form of ). However ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, or unsaturated, like hexene and hexyne. Open-chain compounds, whether straight or branched, and which contain no rings of any type, are always aliphatic. Cyclic compounds can be aliphatic if they are not aromatic. Structure Aliphatic compounds can be saturated, joined by single bonds (alkanes), or unsaturated, with double bonds (alkenes) or triple bonds (alkynes). If other elements (heteroatoms) are bound to the carbon chain, the most common being oxygen, nitrogen, sulfur, and chlorine, it is no longer a hydrocarbon, and therefore no longer an aliphatic compound. The least complex aliphatic compound is methane (CH4). Properties Most aliphatic compounds are flammable, allowing the use of hydrocarbons as fuel, such as methane in Buns ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. Phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring. Nomenclature Usually, a "phenyl group" is synonymous with C6H5− and is represented by the symbol Ph or, archaically, Φ. Benzene is sometimes denoted as PhH. Phenyl groups are generally attached to other atoms or groups. For example, triphenylmethane (Ph3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parylene C Repeat Unit
Parylene is the common name of a polymer whose backbone consists of ''para''- benzenediyl rings –– connected by 1,2-ethanediyl bridges –––. It can be obtained by polymerization of ''para''-xylylene . The name is also used for several polymers with the same backbone, where some hydrogen atoms are replaced by other functional groups. Some of these variants are designated in commerce by letter-number codes such as "parylene C" and "parylene AF-4". Some of these names are registered trademarks in some countries. Coatings of parylene are often applied to electronic circuits and other equipment as electrical insulation, moisture barriers, or protection against corrosion and chemical attack. They are also used to reduce friction, and in medicine to prevent adverse reactions to implanted devices. These coatings are typically applied by chemical vapor deposition in an atmosphere of the monomer ''para''-xylylene. Parylene is considered a "green" polymer because it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
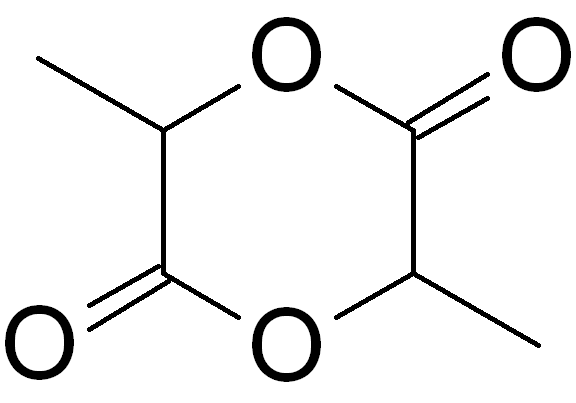
P-xylene
''p''-Xylene ( ''para''-xylene) is an aromatic hydrocarbon. It is one of the three isomers of dimethylbenzene known collectively as xylenes. The ''p-'' stands for ''para-'', indicating that the two methyl groups in ''p''-xylene occupy the diametrically opposite substituent positions 1 and 4. It is in the positions of the two methyl groups, their arene substitution pattern, that it differs from the other isomers, ''o''-xylene and ''m''-xylene. All have the same chemical formula C6H4(CH3)2. All xylene isomers are colorless and highly flammable. The odor threshold of ''p''-xylene is 0.62 parts per million (ppm). Production The production of ''p''-xylene is industrially significant, with annual demand estimated at 37 million tons in 2014, and still on the increase. ''p''-Xylene is produced by catalytic reforming of petroleum naphtha as part of the BTX aromatics (benzene, toluene and the xylene isomers) extracted from the catalytic reformate. The ''p''-xylene is then separated out ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |