|
Pandemonium Effect
The pandemonium effect is a problem that may appear when high-resolution detectors (usually germanium semiconductor detectors) are used in beta decay studies. It can affect the correct determination of the feeding to the different levels of the decay product, daughter nucleus. It was first introduced in 1977. Context Typically, when a parent nucleus beta-decays into its daughter, there is some final energy available which is shared between the final products of the decay. This is called the Q value (nuclear science), ''Q'' value of the beta decay (''Qβ''). The daughter nucleus doesn't necessarily end up in the ground state after the decay, this only happens when the other products have taken all the available energy with them (usually as kinetic energy). So, in general, the daughter nucleus keeps an amount of the available energy as excitation energy and ends up in an excited state associated to some energy level, as seen in the picture. The daughter nucleus can only stay in that e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schematic Diagram Showing The Pandemonium Effect
A schematic, or schematic diagram, is a designed representation of the elements of a system using abstract, graphic symbols rather than realistic pictures. A schematic usually omits all details that are not relevant to the key information the schematic is intended to convey, and may include oversimplified elements in order to make this essential meaning easier to grasp, as well as additional organization of the information. For example, a subway map intended for passengers may represent a subway station with a dot. The dot is not intended to resemble the actual station at all but aims to give the viewer information without unnecessary visual clutter. A schematic diagram of a chemical process uses symbols in place of detailed representations of the vessels, piping, valves, pumps, and other equipment that compose the system, thus emphasizing the functions of the individual elements and the interconnections among them and suppresses their physical details. In an electronic circuit d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gamma Spectroscopy
Gamma-ray spectroscopy is the ''qualitative'' study of the energy spectra of gamma-ray sources, such as in the nuclear industry, geochemical investigation, and astrophysics. Gamma-ray spectrometry, on the other hand, is the method used to acquire a ''quantitative'' spectrum measurement. Most radioactive sources produce gamma rays, which are of various energies and intensities. When these emissions are detected and analyzed with a spectroscopy system, a gamma-ray energy spectrum can be produced. A detailed analysis of this spectrum is typically used to determine the identity and quantity of gamma emitters present in a gamma source, and is a vital tool in radiometric assay. The gamma spectrum is characteristic of the gamma-emitting nuclides contained in the source, just like in an optical spectrometer, the optical spectrum is characteristic of the material contained in a sample. Gamma ray characteristics Gamma rays are the highest-energy form of electromagnetic radiation, being ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gamma-ray Spectrometer
A gamma-ray spectrometer (GRS) is an instrument for measuring the distribution (or spectrum—see Gamma spectroscopy#Scintillation detectors, figure) of the intensity of gamma radiation versus the energy of each photon. The study and analysis of gamma-ray spectra for scientific and technical use is called gamma spectroscopy, and gamma-ray spectrometers are the instruments which observe and collect such data. Because the energy of each photon of EM radiation is proportional to its frequency, gamma rays have sufficient energy that they are typically observed by counting individual photons. Some notable gamma-ray spectrometers are Gammasphere, AGATA (gamma-ray detector), AGATA, and Argonne Tandem Linear Accelerator System, GRETINA. Gamma-ray spectroscopy Atomic atomic nucleus, nuclei have an energy-level structure somewhat analogous to the energy levels of atoms, so that they may emit (or absorb) photons of particular energies, much as atoms do, but at energies that are thousands ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
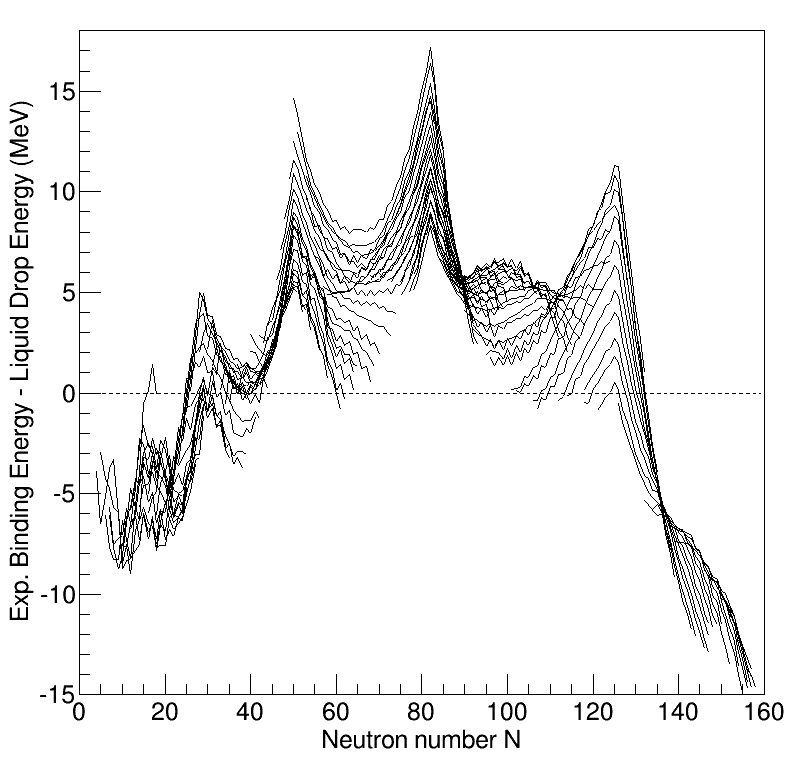
Nuclear Structure
Understanding the structure of the atomic nucleus is one of the central challenges in nuclear physics. Models The cluster model The cluster model describes the nucleus as a molecule-like collection of proton-neutron groups (e.g., alpha particles) with one or more valence neutrons occupying molecular orbitals. The liquid drop model The liquid drop model is one of the first models of nuclear structure, proposed by Carl Friedrich von Weizsäcker in 1935. It describes the nucleus as a semiclassical fluid made up of neutrons and protons, with an internal repulsive electrostatic force proportional to the number of protons. The quantum mechanical nature of these particles appears via the Pauli exclusion principle, which states that no two nucleons of the same kind can be at the same state. Thus the fluid is actually what is known as a Fermi liquid. In this model, the binding energy of a nucleus with Z protons and N neutrons is given by :E_ = a_ A - a_ A^ - a_ \frac - a_ \frac - ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Reactor Technology
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction. They are used for commercial electricity, marine propulsion, weapons production and research. Fissile nuclei (primarily uranium-235 or plutonium-239) absorb single neutrons and split, releasing energy and multiple neutrons, which can induce further fission. Reactors stabilize this, regulating neutron absorbers and moderators in the core. Fuel efficiency is exceptionally high; low-enriched uranium is 120,000 times more energy dense than coal. Heat from nuclear fission is passed to a working fluid coolant. In commercial reactors, this drives turbines and electrical generator shafts. Some reactors are used for district heating, and isotope production for medical and industrial use. Following the 1938 discovery of fission, many countries initiated military nuclear research programs. Early subcritical experiments probed neutronics. In 1942, the first artificial critical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decay Heat
Decay heat is the heat released as a result of radioactive decay. This heat is produced as an effect of radiation on materials: the energy of the alpha particle, alpha, Beta particle, beta or gamma radiation is converted into the thermal movement of atoms. Decay heat occurs naturally from decay of long-lived radioisotopes that are primordially present from the Earth's formation. In nuclear reactor engineering, decay heat continues to be generated after the reactor has been shut down (see SCRAM and nuclear chain reactions) and power generation has been suspended. The decay of the short-lived radioisotopes such as iodine-131 created in fission continues at high power for a time after shutdown (nuclear reactor), shut down. The major source of heat production in a newly shut down reactor is due to the beta decay of new radioactive elements recently produced from fission fragments in the fission process. Quantitatively, at the moment of reactor shutdown, decay heat from these radioact ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scintillation Counter
A scintillation counter is an instrument for detecting and measuring ionizing radiation by using the Electron excitation, excitation effect of incident radiation on a Scintillation (physics), scintillating material, and detecting the resultant light pulses. It consists of a scintillator which generates photons in response to incident radiation, a sensitive photodetector (usually a photomultiplier tube (PMT), a charge-coupled device (CCD) camera, or a photodiode), which converts the light to an electrical signal and electronics to process this signal. Scintillation counters are widely used in radiation protection, assay of radioactive materials and physics research because they can be made inexpensively yet with good quantum efficiency, and can measure both the intensity and the Electronvolt, energy of incident radiation. History The first electronic scintillation counter was invented in 1944 by Samuel Curran, Sir Samuel Curran whilst he was working on the Manhattan Project at th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Total Absorption Spectroscopy
Total absorption spectroscopy is a measurement technique that allows the measurement of the gamma radiation emitted in the different nuclear gamma transitions that may take place in the daughter nucleus after its unstable parent has decayed by means of the beta decay process. This technique can be used for beta decay studies related to beta feeding measurements ''within the full decay energy window'' for nuclei far from stability. It is implemented with a special type of detector, the "''total absorption spectrometer''" (TAS), made of a scintillator crystal that almost completely surrounds the activity to be measured, covering a solid angle of approximately 4π. Also, in an ideal case, it should be thick enough to have a peak efficiency close to 100%, in this way its total efficiency is also very close to 100% (this is one of the reasons why it is called "total" absorption spectroscopy). Finally, it should be blind to any other type of radiation. The gamma rays produced in the decay ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Volt
In physics, an electronvolt (symbol eV), also written electron-volt and electron volt, is the measure of an amount of kinetic energy gained by a single electron accelerating through an electric potential difference of one volt in vacuum. When used as a unit of energy, the numerical value of 1 eV in joules (symbol J) is equal to the numerical value of the charge of an electron in coulombs (symbol C). Under the 2019 revision of the SI, this sets 1 eV equal to the exact value Historically, the electronvolt was devised as a standard unit of measure through its usefulness in electrostatic particle accelerator sciences, because a particle with electric charge ''q'' gains an energy after passing through a voltage of ''V''. Definition and use An electronvolt is the amount of energy gained or lost by a single electron when it moves through an electric potential difference of one volt. Hence, it has a value of one volt, which is , multiplied by the elementary charge Therefore ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selection Rule
In physics and chemistry, a selection rule, or transition rule, formally constrains the possible transitions of a system from one quantum state to another. Selection rules have been derived for electromagnetic transitions in molecules, in atoms, in atomic nucleus, atomic nuclei, and so on. The selection rules may differ according to the technique used to observe the transition. The selection rule also plays a role in chemical reactions, where some are formally spin-forbidden reactions, that is, reactions where the spin state changes at least once from Reagent, reactants to Product (chemistry), products. In the following, mainly atomic and molecular transitions are considered. Overview In quantum mechanics the basis for a spectroscopic selection rule is the value of the ''transition moment integral'' :m_ = \int \psi_1^* \, \mu \, \psi_2 \, \mathrm\tau, where \psi_1 and \psi_2 are the wave functions of the two states, "state 1" and "state 2", involved in the transition, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semiconductor Detector
In ionizing radiation detection physics, a semiconductor detector is a device that uses a semiconductor (usually silicon or germanium) to measure the effect of incident charged particles or photons. Semiconductor detectors find broad application for radiation protection, gamma spectroscopy, gamma and x-ray spectroscopy, X-ray spectrometry, and as particle detectors. Detection mechanism In semiconductor detectors, ionizing radiation is measured by the number of charge carriers set free in the detector material which is arranged between two electrodes, by the radiation. Ionizing radiation produces free electrons and electron holes. The number of electron-hole pairs is proportional to the energy of the radiation to the semiconductor. As a result, a number of electrons are transferred from the valence band to the conduction band, and an equal number of holes are created in the valence band. Under the influence of an electric field, electrons and holes travel to the electrodes, wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |