|
PF-04457845
JZP150 (formerly PF-04457845) is an inhibitor of the enzyme fatty acid amide hydrolase (FAAH), with an IC50 of 7.2nM, and both analgesic and antiinflammatory effects in animal studies comparable to naproxen. JZP150 is currently being developed by Jazz Pharmaceuticals and is in a Phase 2 trial in the US for PTSD. See also * FAAH inhibitor ** 4-Nonylphenylboronic acid ** LY-2183240 ** URB-597 ** BIA 10-2474, problems in phase 1 References {{Cannabinoids Enzyme inhibitors Trifluoromethyl compounds Pyridazines Pyridines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BIA 10-2474
BIA 10-2474 is an experimental fatty acid amide hydrolase inhibitor developed by the Portuguese pharmaceutical company Bial-Portela & Ca. SA. It interacts with the human endocannabinoid system. The drug was in development for the treatment of a range of different medical conditions from anxiety disorder to Parkinson's disease, also for the treatment of chronic pain of multiple sclerosis, cancer, hypertension or the treatment of obesity. A clinical trial with this drug was underway in Rennes, France, in January 2016, in which serious adverse events occurred affecting five participants, including the death of one man. The underlying mechanism that caused the acute neurotoxicity of this molecule remains unknown. Structure and action The chemical name of BIA-10-2474 is 3-(1-(cyclohexyl(methyl)carbamoyl)-1H-imidazol-4-yl)pyridine 1-oxide. BIA-10-2474 is a long-acting inhibitor of fatty acid amide hydrolase (FAAH) that increases levels of the neurotransmitter anandamide in the centra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fatty Acid Amide Hydrolase
Fatty acid amide hydrolase or FAAH (, oleamide hydrolase, anandamide amidohydrolase) is a member of the serine hydrolase family of enzymes. It was first shown to break down anandamide in 1993. In humans, it is encoded by the gene ''FAAH''.; Function FAAH is an integral membrane hydrolase with a single ''N''-terminal transmembrane domain. ''In vitro'', FAAH has esterase and amidase activity. ''In vivo'', FAAH is the principal catabolic enzyme for a class of bioactive lipids called the fatty acid amides (FAAs). Members of the FAAs include: * Anandamide (''N''-arachidonoylethanolamine), an endocannabinoid * 2-arachidonoylglycerol (2-AG), an endocannabinoid. * Other ''N''-acylethanolamines, such as ''N''-oleoylethanolamine and ''N''-palmitoylethanolamine * The sleep-inducing lipid oleamide * The ''N''-acyltaurines, which are agonists of the transient receptor potential (TRP) family of calcium channels. ''FAAH'' knockout mice display highly elevated (>15-fold) levels of ''N'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FAAH Inhibitor
Fatty acid amide hydrolase or FAAH (, oleamide hydrolase, anandamide amidohydrolase) is a member of the serine hydrolase family of enzymes. It was first shown to break down anandamide in 1993. In humans, it is encoded by the gene ''FAAH''.; Function FAAH is an integral membrane hydrolase with a single ''N''-terminal transmembrane domain. ''In vitro'', FAAH has esterase and amidase activity. ''In vivo'', FAAH is the principal catabolic enzyme for a class of bioactive lipids called the fatty acid amides (FAAs). Members of the FAAs include: * Anandamide (''N''-arachidonoylethanolamine), an endocannabinoid *2-arachidonoylglycerol (2-AG), an endocannabinoid. * Other ''N''-acylethanolamines, such as ''N''-oleoylethanolamine and ''N''-palmitoylethanolamine * The sleep-inducing lipid oleamide * The ''N''-acyltaurines, which are agonists of the transient receptor potential (TRP) family of calcium channels. ''FAAH'' knockout mice display highly elevated (>15-fold) levels of ''N''- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LY-2183240
LY-2183240 is a drug which acts both as a potent inhibitor of the reuptake of the endocannabinoid anandamide and as an inhibitor of fatty acid amide hydrolase (FAAH), the primary enzyme responsible for degrading anandamide. This leads to markedly elevated anandamide levels in the brain, and LY-2183240 has been shown to produce both analgesic and anxiolytic effects in animal models. While LY-2183240 is a potent inhibitor of FAAH, it has relatively poor selectivity and also inhibits several other enzyme side targets. Consequently, it was never developed for clinical use, though it remains widely used in research, and has also been sold as a designer drug. See also *Endocannabinoid reuptake inhibitor * FAAH inhibitor ** BIA 10-2474 ** PF-04457845 ** URB-597 URB597 (KDS-4103) is a relatively selective and irreversible inhibitor of the enzyme fatty acid amide hydrolase (FAAH). FAAH is the primary degradatory enzyme for the endocannabinoid anandamide and, as such, inhibition of FAAH l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
URB-597
URB597 (KDS-4103) is a relatively selective and irreversible inhibitor of the enzyme fatty acid amide hydrolase (FAAH). FAAH is the primary degradatory enzyme for the endocannabinoid anandamide and, as such, inhibition of FAAH leads to an accumulation of anandamide in the CNS and periphery where it activates cannabinoid receptors. URB597 has been found to elevate anandamide levels and have activity against neuropathic pain in a mouse model. Preclinical studies have shown FAAH inhibitors to increase BDNF levels in the hippocampus and prefrontal cortex, highlighting their potential in addiction treatment as "enviromimetics". Indeed, Chauvet et al. found that chronic URB597 administration in rats "significantly reduces cocaine-seeking behaviour and cue- and stress-induced relapse". URB597 was at one point being developed by Kadmus Pharmaceuticals, Inc. for clinical trials in humans. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analgesic
An analgesic drug, also called simply an analgesic (American English), analgaesic (British English), pain reliever, or painkiller, is any member of the group of drugs used to achieve relief from pain (that is, analgesia or pain management). It is typically used to induce cooperation with a medical procedure. Analgesics are conceptually distinct from anesthetics, which temporarily reduce, and in some instances eliminate, sensation, although analgesia and anesthesia are neurophysiologically overlapping and thus various drugs have both analgesic and anesthetic effects. Analgesic choice is also determined by the type of pain: For neuropathic pain, traditional analgesics are less effective, and there is often benefit from classes of drugs that are not normally considered analgesics, such as tricyclic antidepressants and anticonvulsants. Various analgesics, such as many NSAIDs, are available over the counter in most countries, whereas various others are prescription drugs owing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antiinflammatory
Anti-inflammatory is the property of a substance or treatment that reduces inflammation or swelling. Anti-inflammatory drugs, also called anti-inflammatories, make up about half of analgesics. These drugs remedy pain by reducing inflammation as opposed to opioids, which affect the central nervous system to block pain signaling to the brain. Nonsteroidal anti-inflammatory drugs Nonsteroidal anti-inflammatory drugs (NSAIDs) alleviate pain by counteracting the cyclooxygenase (COX) enzyme. On its own, COX enzyme synthesizes prostaglandins, creating inflammation. In whole, the NSAIDs prevent the prostaglandins from ever being synthesized, reducing or eliminating the inflammation and resulting pain. Some common examples of NSAIDs are aspirin, ibuprofen, and naproxen. The newer specific COX-inhibitors are not classified together with the traditional NSAIDs, even though they presumably share the same mode of action. On the other hand, there are analgesics that are commonly associat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naproxen
Naproxen is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain, menstrual cramps, inflammatory diseases such as rheumatoid arthritis, gout and fever. It is taken orally. It is available in immediate and delayed release formulations. Onset of effects is within an hour and lasts for up to twelve hours. Common side effects include dizziness, headache, bruising, allergic reactions, heartburn, and stomach pain. Severe side effects include an increased risk of heart disease, stroke, gastrointestinal bleeding, and stomach ulcers. The heart disease risk may be lower than with other NSAIDs. It is not recommended in people with kidney problems. Use is not recommended in the third trimester of pregnancy. Naproxen is a nonselective COX inhibitor. As an NSAID, naproxen appears to exert its anti-inflammatory action by reducing the production of inflammatory mediators called prostaglandins. It is metabolized by the liver to inactive metabolites. Naproxen was patented in 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-Nonylphenylboronic Acid
4-Nonylphenylboronic acid is a potent and selective inhibitor of the enzyme fatty acid amide hydrolase (FAAH), with an IC50 of 9.1nM, and 870x selectivity for FAAH over the related enzyme MAGL, which it inhibits with an IC50 of 7900nM. It is also a weaker inhibitor of the enzymes endothelial lipase and lipoprotein lipase, with values of 100 nM and 1400 nM respectively. See also * IDFP IDFP is an organophosphorus compound related to the nerve agent sarin. Like sarin, IDFP is an irreversible inhibitor for a number of different enzymes that normally serve to break down neurotransmitters, however the long alkyl chain of IDFP make ... * LY-2183240 * URB-597 References {{DEFAULTSORT:Nonylphenylboronic acid, 4- Enzyme inhibitors Boronic acids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme Inhibitors
An enzyme inhibitor is a molecule that binds to an enzyme and blocks its activity. Enzymes are proteins that speed up chemical reactions necessary for life, in which substrate molecules are converted into products. An enzyme facilitates a specific chemical reaction by binding the substrate to its active site, a specialized area on the enzyme that accelerates the most difficult step of the reaction. An enzyme inhibitor stops ("inhibits") this process, either by binding to the enzyme's active site (thus preventing the substrate itself from binding) or by binding to another site on the enzyme such that the enzyme's catalysis of the reaction is blocked. Enzyme inhibitors may bind reversibly or irreversibly. Irreversible inhibitors form a chemical bond with the enzyme such that the enzyme is inhibited until the chemical bond is broken. By contrast, reversible inhibitors bind non-covalently and may spontaneously leave the enzyme, allowing the enzyme to resume its function. Reve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
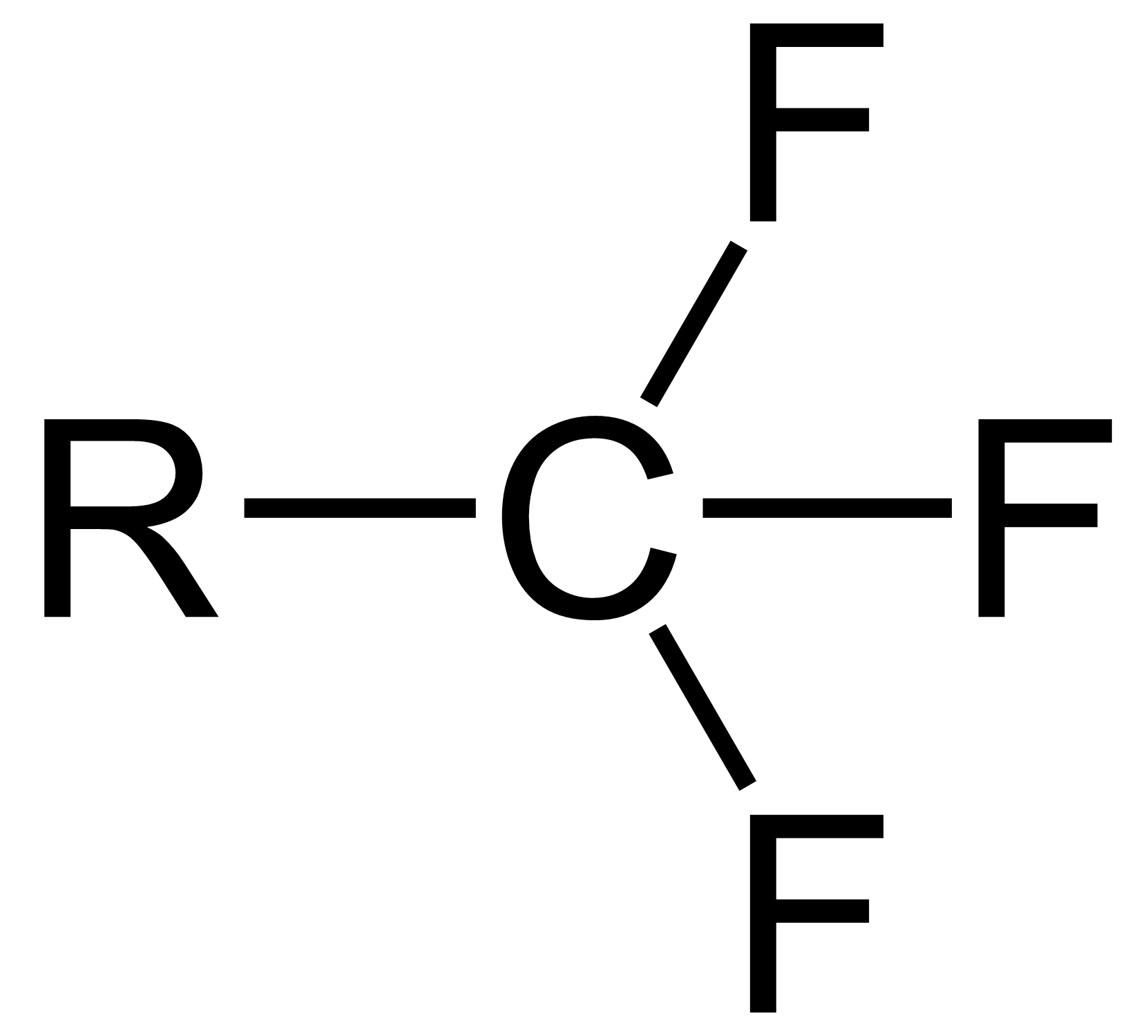
Trifluoromethyl Compounds
The trifluoromethyl group is a functional group that has the formula -CF3. The naming of is group is derived from the methyl group (which has the formula -CH3), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluoromethane H–, 1,1,1-trifluoroethane –, and hexafluoroacetone –CO–. Compounds with this group are a subclass of the organofluorines. Properties The trifluoromethyl group has a significant electronegativity that is often described as being intermediate between the electronegativities of fluorine and chlorine. For this reason, trifluoromethyl-substituted compounds are often strong acids, such as trifluoromethanesulfonic acid and trifluoroacetic acid. Conversely, the trifluoromethyl group lowers the basicity of compounds like trifluoroethanol. Uses The trifluoromethyl group occurs in certain pharmaceuticals, drugs, and abiotically synthesized natural fluorocarbon based compounds. The medicinal use of the trifloromethyl group dates from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridazines
Pyridazine is an aromatic, heterocyclic, organic compound with the molecular formula . It contains a six-membered ring with two adjacent nitrogen atoms. It is a colorless liquid with a boiling point of 208 °C. It is isomeric with two other diazine () rings, pyrimidine and pyrazine. Occurrence Pyridazines are rare in nature, possibly reflecting the scarcity of naturally occurring hydrazines, common building blocks for the synthesis of these heterocycles. The pyridazine structure is a popular pharmacophore which is found within a number of herbicides such as credazine, pyridafol and pyridate. It is also found within the structure of several drugs such as cefozopran, cadralazine, minaprine, pipofezine, and hydralazine. Synthesis In the course of his classic investigation on the Fischer indole synthesis, Emil Fischer prepared the first pyridazine via the condensation of phenylhydrazine and levulinic acid. The parent heterocycle was first prepared by oxidation of benz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |