|
Otto Cycle
An Otto cycle is an idealized thermodynamic cycle that describes the functioning of a typical spark ignition piston engine. It is the thermodynamic cycle most commonly found in automobile engines. The Otto cycle is a description of what happens to a gas as it is subjected to changes of pressure, temperature, volume, addition of heat, and removal of heat. The gas that is subjected to those changes is called the system. The system, in this case, is defined to be the fluid (gas) within the cylinder. By describing the changes that take place within the system, it will also describe in inverse, the system's effect on the environment. In the case of the Otto cycle, the effect will be to produce enough net work from the system so as to propel an automobile and its occupants in the environment. The Otto cycle is constructed from: :Top and bottom of the loop: a pair of quasi-parallel and isentropic processes (frictionless, adiabatic reversible). :Left and right sides of the loop: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicolaus Otto
Nicolaus August Otto (10 June 1832, Holzhausen an der Haide, Nassau – 26 January 1891, Cologne) was a German engineer who successfully developed the compressed charge internal combustion engine which ran on petroleum gas and led to the modern internal combustion engine. The Association of German Engineers (VDI) created DIN standard 1940 which says "Otto Engine: internal combustion engine in which the ignition of the compressed fuel-air mixture is initiated by a timed spark", which has been applied to all engines of this type since. Biography Nicolaus August Otto was born on 10 June 1832 in Holzhausen an der Haide, Germany. He was the youngest of six children. His father died in 1832. He began school in 1838. After six years of good performance he moved to the high school in Langenschwalbach until 1848. He did not complete his studies but was cited for good performance. His main interest in school had been in science and technology but he graduated after three years as a bu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piston Engines
A reciprocating engine, also often known as a piston engine, is typically a heat engine that uses one or more reciprocating pistons to convert high temperature and high pressure into a rotating motion. This article describes the common features of all types. The main types are: the internal combustion engine, used extensively in motor vehicles; the steam engine, the mainstay of the Industrial Revolution; and the Stirling engine for niche applications. Internal combustion engines are further classified in two ways: either a spark-ignition (SI) engine, where the spark plug initiates the combustion; or a compression-ignition (CI) engine, where the air within the cylinder is compressed, thus heating it, so that the heated air ignites fuel that is injected then or earlier.''Thermodynamics: An Engineering Approach'' by Yunus A. Cengal and Michael A. Boles Common features in all types There may be one or more pistons. Each piston is inside a cylinder, into which a gas is intro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intercooler
An intercooler is a heat exchanger used to cool a gas after compression. Often found in turbocharged engines, intercoolers are also used in air compressors, air conditioners, refrigeration and gas turbines. Internal combustion engines Most commonly used with turbocharged engines, an intercooler is used to counteract the heat of compression and heat soak in the pressurised intake air. By reducing the temperature of the intake air, the air becomes denser (allowing more fuel to be injected, resulting in increased power) and less likely to suffer from pre-ignition or knocking. Additional cooling can be provided by externally spraying a fine mist onto the intercooler surface, or even into the intake air itself, to further reduce intake charge temperature through evaporative cooling. Intercoolers can vary dramatically in size, shape and design, depending on the performance and space requirements of the system. Many passenger cars use either ''front-mounted intercoolers'' l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
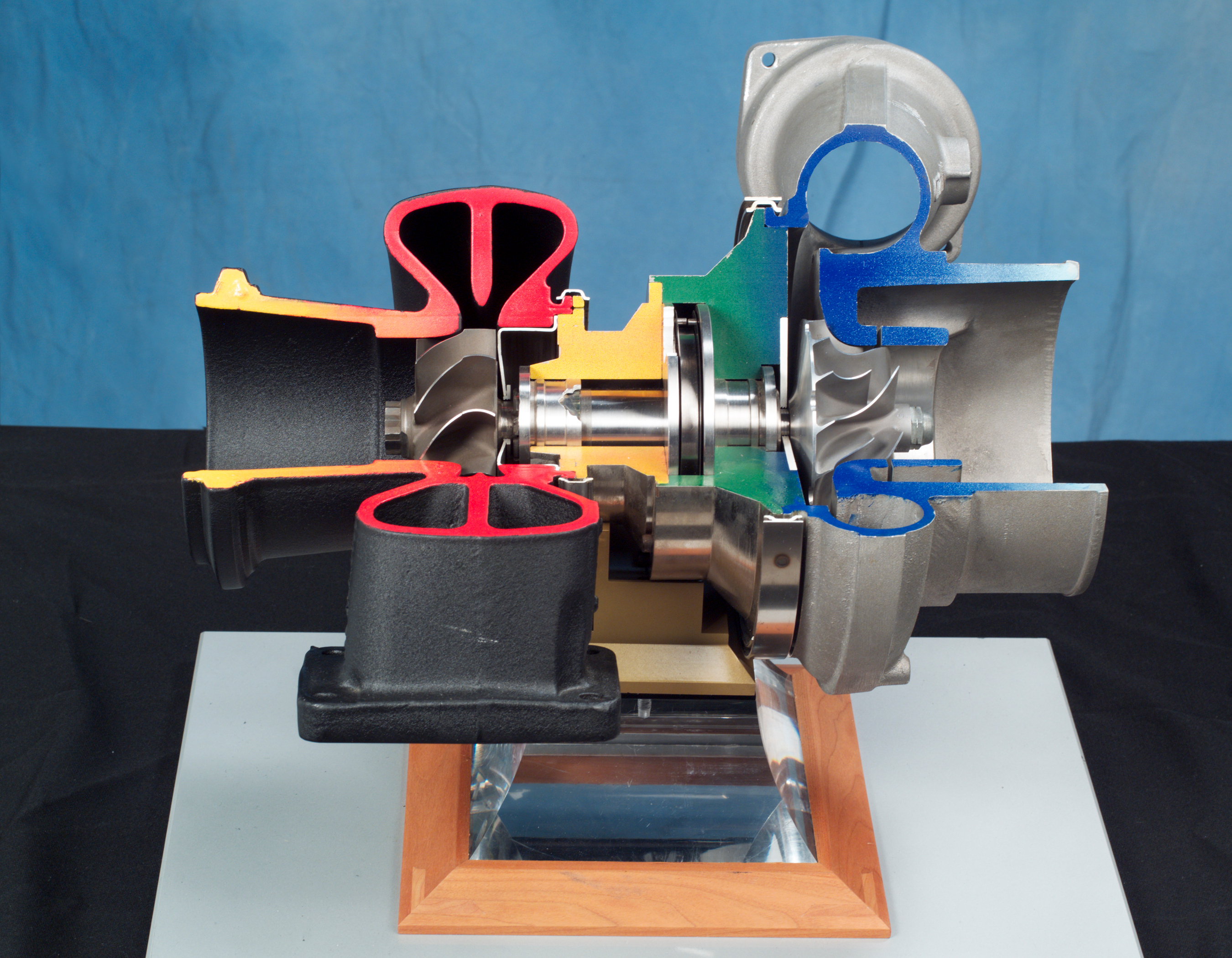
Turbocharger
In an internal combustion engine, a turbocharger (often called a turbo) is a forced induction device that is powered by the flow of exhaust gases. It uses this energy to compress the intake gas, forcing more air into the engine in order to produce more power for a given displacement. The current categorisation is that a turbocharger is powered by the kinetic energy of the exhaust gasses, whereas a supercharger is mechanically powered (usually by a belt from the engine's crankshaft). However, up until the mid-20th century, a turbocharger was called a "turbosupercharger" and was considered a type of supercharger. History Prior to the invention of the turbocharger, |
Engine Knocking
In spark ignition internal combustion engines, knocking (also knock, detonation, spark knock, pinging or pinking) occurs when combustion of some of the air/fuel mixture in the cylinder does not result from propagation of the flame front ignited by the spark plug, but one or more pockets of air/fuel mixture explode outside the envelope of the normal combustion front. The fuel-air charge is meant to be ignited by the spark plug only, and at a precise point in the piston's stroke. Knock occurs when the peak of the combustion process no longer occurs at the optimum moment for the four-stroke cycle. The shock wave creates the characteristic metallic "pinging" sound, and cylinder pressure increases dramatically. Effects of engine knocking range from inconsequential to completely destructive. Knocking should not be confused with pre-ignition—they are two separate events. However, pre-ignition can be followed by knocking. The phenomenon of detonation was described in November 1914 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Gas Constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per amount of substance, i.e. the pressure–volume product, rather than energy per temperature increment per ''particle''. The constant is also a combination of the constants from Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. It is a physical constant that is featured in many fundamental equations in the physical sciences, such as the ideal gas law, the Arrhenius equation, and the Nernst equation. The gas constant is the constant of proportionality that relates the energy scale in physics to the temperature scale and the scale used for amount of substance. Thus, the value of the gas constant ultimately derives from historical decisions and accidents in the setting of units of energy, temperature and amount of substanc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isentropic Process
In thermodynamics, an isentropic process is an idealized thermodynamic process that is both adiabatic and reversible. The work transfers of the system are frictionless, and there is no net transfer of heat or matter. Such an idealized process is useful in engineering as a model of and basis of comparison for real processes. This process is idealized because reversible processes do not occur in reality; thinking of a process as both adiabatic and reversible would show that the initial and final entropies are the same, thus, the reason it is called isentropic (entropy does not change). Thermodynamic processes are named based on the effect they would have on the system (ex. isovolumetric: constant volume, isenthalpic: constant enthalpy). Even though in reality it is not necessarily possible to carry out an isentropic process, some may be approximated as such. The word "isentropic" can be interpreted in another way, since its meaning is deducible from its etymology. It means a pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ideal Gas
An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics. The requirement of zero interaction can often be relaxed if, for example, the interaction is perfectly elastic or regarded as point-like collisions. Under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules (or atoms for monatomic gas) play the role of the ideal particles. Many gases such as nitrogen, oxygen, hydrogen, noble gases, some heavier gases like carbon dioxide and mixtures such as air, can be treated as ideal gases within reasonable tolerances over a considerable parameter range around standard temperature and pressure. Generally, a gas behaves more like an ideal gas at higher temperature and lower pressu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K). Heat capacity is an extensive property. The corresponding intensive property is the specific heat capacity, found by dividing the heat capacity of an object by its mass. Dividing the heat capacity by the amount of substance in moles yields its molar heat capacity. The volumetric heat capacity measures the heat capacity per volume. In architecture and civil engineering, the heat capacity of a building is often referred to as its thermal mass. Definition Basic definition The heat capacity of an object, denoted by C, is the limit : C = \lim_\frac, where \Delta Q is the amount of heat that must be added to the object (of mass ''M'') in order to raise its temperature by \Delta T. The value of this parameter usually varies considerably depending on the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Compression Ratio
The compression ratio is the ratio between the volume of the cylinder and combustion chamber in an internal combustion engine at their maximum and minimum values. A fundamental specification for such engines, it is measured two ways: the static compression ratio, calculated based on the relative volumes of the combustion chamber and the cylinder when the piston is at the bottom of its stroke, and the volume of the combustion chamber when the piston is at the top of its stroke. The dynamic compression ratio is a more advanced calculation which also takes into account gasses entering and exiting the cylinder during the compression phase. Effect and typical ratios A high compression ratio is desirable because it allows an engine to extract more mechanical energy from a given mass of air–fuel mixture due to its higher thermal efficiency. This occurs because internal combustion engines are heat engines, and higher compression ratios permit the same combustion temperature to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
OTTO CYCLE
An Otto cycle is an idealized thermodynamic cycle that describes the functioning of a typical spark ignition piston engine. It is the thermodynamic cycle most commonly found in automobile engines. The Otto cycle is a description of what happens to a gas as it is subjected to changes of pressure, temperature, volume, addition of heat, and removal of heat. The gas that is subjected to those changes is called the system. The system, in this case, is defined to be the fluid (gas) within the cylinder. By describing the changes that take place within the system, it will also describe in inverse, the system's effect on the environment. In the case of the Otto cycle, the effect will be to produce enough net work from the system so as to propel an automobile and its occupants in the environment. The Otto cycle is constructed from: :Top and bottom of the loop: a pair of quasi-parallel and isentropic processes (frictionless, adiabatic reversible). :Left and right sides of the loop: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |