|
Organoargon Chemistry
Argon compounds, the chemical compounds that contain the element argon, are rarely encountered due to the inertness of the argon atom. However, compounds of argon have been detected in inert gas matrix isolation, cold gases, and plasmas, and molecular ions containing argon have been made and also detected in space. One solid interstitial compound of argon, Ar1C60 is stable at room temperature. Ar1C60 was discovered by the CSIRO. Argon ionises at 15.76 eV, which is higher than hydrogen, but lower than helium, neon or fluorine. Molecules containing argon can be van der Waals molecules held together very weakly by London dispersion forces. Ionic molecules can be bound by charge induced dipole interactions. With gold atoms there can be some covalent interaction. Several boron-argon bonds with significant covalent interactions have been also reported. Experimental methods used to study argon compounds have included inert gas matrices, infrared spectroscopy to study stretching and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abundant as water vapor (which averages about 4000 ppmv, but varies greatly), 23 times as abundant as carbon dioxide (400 ppmv), and more than 500 times as abundant as neon (18 ppmv). Argon is the most abundant noble gas in Earth's crust, comprising 0.00015% of the crust. Nearly all of the argon in Earth's atmosphere is radiogenic argon-40, derived from the decay of potassium-40 in Earth's crust. In the universe, argon-36 is by far the most common argon isotope, as it is the most easily produced by stellar nucleosynthesis in supernovas. The name "argon" is derived from the Greek word , neuter singular form of meaning 'lazy' or 'inactive', as a reference to the fact that the element undergoes almost no chemical reactions. The complete octe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Effective Core Potential
In physics, a pseudopotential or effective potential is used as an approximation for the simplified description of complex systems. Applications include atomic physics and neutron scattering. The pseudopotential approximation was first introduced by Hans Hellmann in 1934. Atomic physics The pseudopotential is an attempt to replace the complicated effects of the motion of the core (i.e. non- valence) electrons of an atom and its nucleus with an effective potential, or pseudopotential, so that the Schrödinger equation contains a modified effective potential term instead of the Coulombic potential term for core electrons normally found in the Schrödinger equation. The pseudopotential is an effective potential constructed to replace the atomic all-electron potential (full-potential) such that core states are eliminated ''and'' the valence electrons are described by pseudo-wavefunctions with significantly fewer nodes. This allows the pseudo-wavefunctions to be described with far ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Icosahedral
In geometry, an icosahedron ( or ) is a polyhedron with 20 faces. The name comes and . The plural can be either "icosahedra" () or "icosahedrons". There are infinitely many non- similar shapes of icosahedra, some of them being more symmetrical than others. The best known is the (convex, non- stellated) regular icosahedron—one of the Platonic solids—whose faces are 20 equilateral triangles. Regular icosahedra There are two objects, one convex and one nonconvex, that can both be called regular icosahedra. Each has 30 edges and 20 equilateral triangle faces with five meeting at each of its twelve vertices. Both have icosahedral symmetry. The term "regular icosahedron" generally refers to the convex variety, while the nonconvex form is called a ''great icosahedron''. Convex regular icosahedron The convex regular icosahedron is usually referred to simply as the ''regular icosahedron'', one of the five regular Platonic solids, and is represented by its Schläfli symbol , co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ångström
The angstromEntry "angstrom" in the Oxford online dictionary. Retrieved on 2019-03-02 from https://en.oxforddictionaries.com/definition/angstrom.Entry "angstrom" in the Merriam-Webster online dictionary. Retrieved on 2019-03-02 from https://www.merriam-webster.com/dictionary/angstrom. (, ; , ) or ångström is a metric unit of length equal to m; that is, one ten-billionth ( US) of a metre, a hundred-millionth of a centimetre,Entry "angstrom" in the Oxford English Dictionary, 2nd edition (1986). Retrieved on 2021-11-22 from https://www.oed.com/oed2/00008552. 0.1 nanometre, or 100 picometres. Its symbol is Å, a letter of the Swedish alphabet. The unit is named after the Swedish physicist Anders Jonas Ångström (1814–1874). The angstrom is often used in the natural sciences and technology to express sizes of atoms, molecules, microscopic biological structures, and lengths of chemical bonds, arrangement of atoms in crystals,Arturas Vailionis (2015):Geometry of Crystals Lectur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
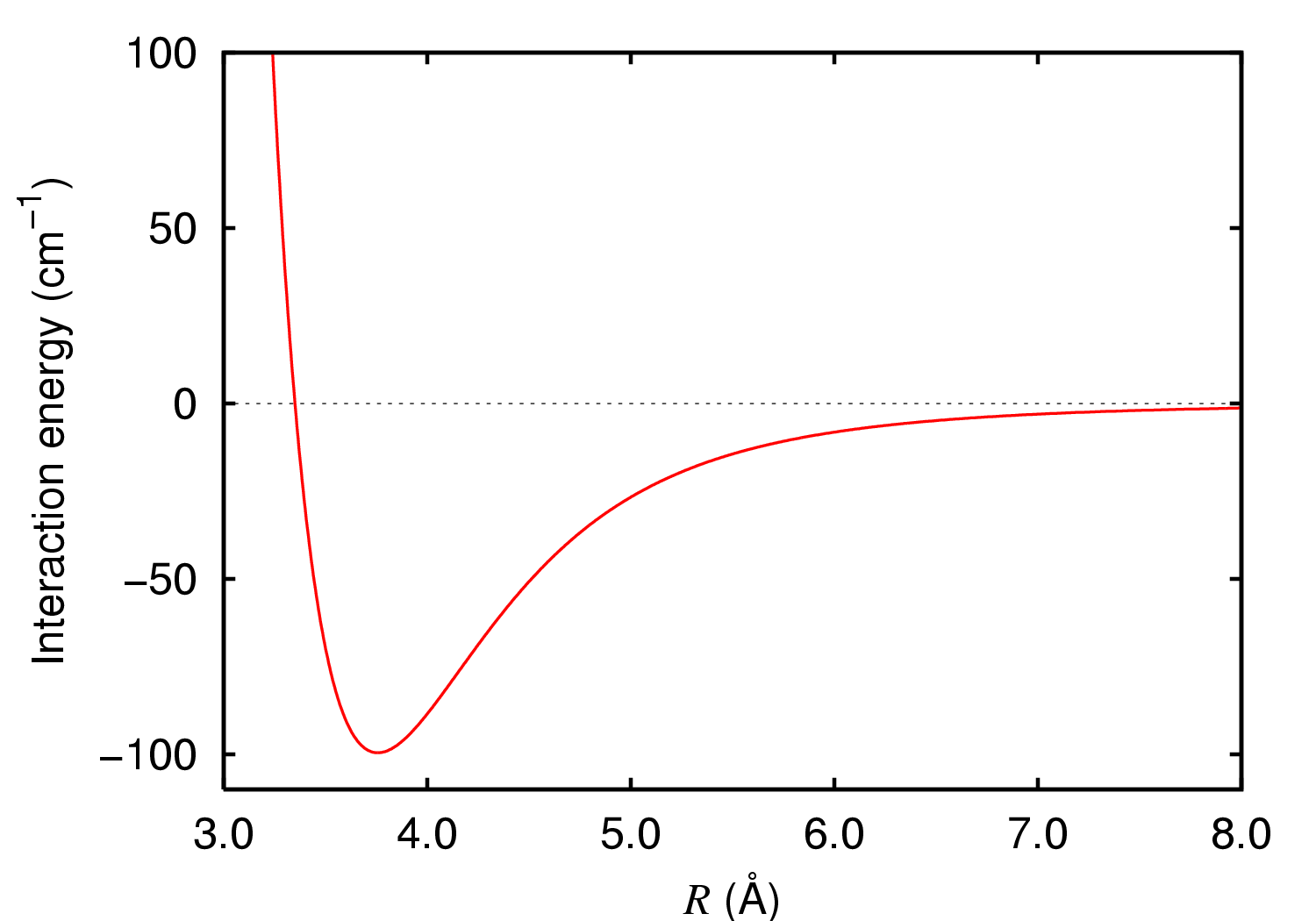
Diargon
Diargon or the argon dimer is a molecule containing two argon atoms. Normally, this is only very weakly bound together by van der Waals forces (a van der Waals molecule). However, in an excited state, or ionised state, the two atoms can be more tightly bound together, with significant spectral features. At cryogenic temperatures, argon gas can have a few percent of diargon molecules. Theory Two argon atoms are attracted together by van der Waals forces when far from each other. When they are close, electrostatic forces repel them. There is a balance point where the van der Waals force matches the opposing repelling force, where energy is at a minimum, represented as the trough in the graph of interaction energy versus distance. This distance is the ground state of the unexcited argon dimer. In a vibrating molecule, the distance between the atoms bounces backwards and forwards from one side of the trough to the other. Faster vibrations will force the state up to higher levels in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Column Density
The area density (also known as areal density, surface density, superficial density, areic density, mass thickness, column density, or density thickness) of a two-dimensional object is calculated as the mass per unit area. The SI derived unit is the kilogram per square metre (kg·m−2). A related '' area number density'' can be defined by replacing mass in by number of particles or other countable quantity. In the paper and fabric industries, it is called grammage and is expressed in grams per square meter (g/m2); for paper in particular, it may be expressed as pounds per ream of standard sizes ("basis ream"). Formulation Area density can be calculated as: \rho_A = \frac or \rho_A = \rho \cdot l where, Column density A special type of area density is called ''column (mass) density'' (also ''columnar mass density''), denoted ''ρ''A or ''σ''. It is the mass of substance per unit area integrated along a path; It is obtained integrating volumetric density \rho ov ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emission Line
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from emission or absorption of light in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used to identify atoms and molecules. These "fingerprints" can be compared to the previously collected ones of atoms and molecules, and are thus used to identify the atomic and molecular components of stars and planets, which would otherwise be impossible. Types of line spectra Spectral lines are the result of interaction between a quantum system (usually atoms, but sometimes molecules or atomic nucleus, atomic nuclei) and a single photon. When a photon has about the right amount of photon energy, energy (which is connected to its frequency) to allow a change in the energy state of the system (in the case of an atom this is usually an electron changing Electron configuration, orbitals), the photon is absorbed. Then the energy will be spontaneously ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crab Nebula
The Crab Nebula (catalogue designations Messier object, M1, New General Catalogue, NGC 1952, Taurus (constellation), Taurus A) is a supernova remnant and pulsar wind nebula in the constellation of Taurus (constellation), Taurus. The common name comes from William Parsons, 3rd Earl of Rosse, who observed the object in 1842 using a telescope and produced a drawing that looked somewhat like a crab. The nebula was discovered by English astronomer John Bevis in 1731, and it corresponds with SN 1054, a bright supernova recorded by Chinese astronomy, Chinese astronomers in 1054. The nebula was the first astronomical object identified that corresponds with a historical supernova explosion. At an apparent magnitude of 8.4, comparable to that of Titan (moon), Saturn's moon Titan, it is not visible to the naked eye but can be made out using binoculars under favourable conditions. The nebula lies in the Perseus Arm of the Milky Way galaxy, at a distance of about from Earth. It has a diame ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydrogen Cation
The dihydrogen cation or hydrogen molecular ion is a cation (positive ion) with formula . It consists of two hydrogen nuclei ( protons) sharing a single electron. It is the simplest molecular ion. The ion can be formed from the ionization of a neutral hydrogen molecule . It is commonly formed in molecular clouds in space, by the action of cosmic rays. The dihydrogen cation is of great historical and theoretical interest because, having only one electron, the equations of quantum mechanics that describe its structure can be solved in a relatively straightforward way. The first such solution was derived by Ø. Burrau in 1927, just one year after the wave theory of quantum mechanics was published. Physical properties Bonding in can be described as a covalent one-electron bond, which has a formal bond order of one half. The ground state energy of the ion is -0.597 Hartree. Isotopologues The dihydrogen cation has six isotopologues, that result from replacement of one o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,00 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Hydrogen
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the baryonic mass of the universe. In everyday life on Earth, isolated hydrogen atoms (called "atomic hydrogen") are extremely rare. Instead, a hydrogen atom tends to combine with other atoms in compounds, or with another hydrogen atom to form ordinary (diatomic) hydrogen gas, H2. "Atomic hydrogen" and "hydrogen atom" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms). Atomic spectroscopy shows that there is a discrete infinite set of states in which a hydrogen (or any) atom can exist, contrary to the predictions of classical physics. Attempts to develop a theoreti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Argonium
Argonium (also called the argon hydride cation, the hydridoargon(1+) ion, or protonated argon; chemical formula ArH+) is a cation combining a proton and an argon atom. It can be made in an electric discharge, and was the first noble gas molecular ion to be found in interstellar space. Properties Argonium is isoelectronic with hydrogen chloride. Its dipole moment is 2.18 D for the ground state. The binding energy is 369 kJ mol−1 (2.9 eV). This is smaller than that of and many other protonated species, but more than that of . Rotationless radiative lifetimes of different vibrational states vary with isotope and become shorter for the more rapid high-energy vibrations: : The force constant in the bond is calculated at 3.88 mdyne/Å2. Reactions *ArH+ + H2 → Ar + *ArH+ + C → Ar + CH+ *ArH+ + N → Ar + NH+ *ArH+ + O → Ar + OH+ *ArH+ + CO → Ar + COH+ But the reverse reaction happens: *Ar + → ArH+ + H. *Ar + → *ArH+ + H2 Ar+ + H2 h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |