Diargon on:
[Wikipedia]
[Google]
[Amazon]
Diargon or the argon dimer is a
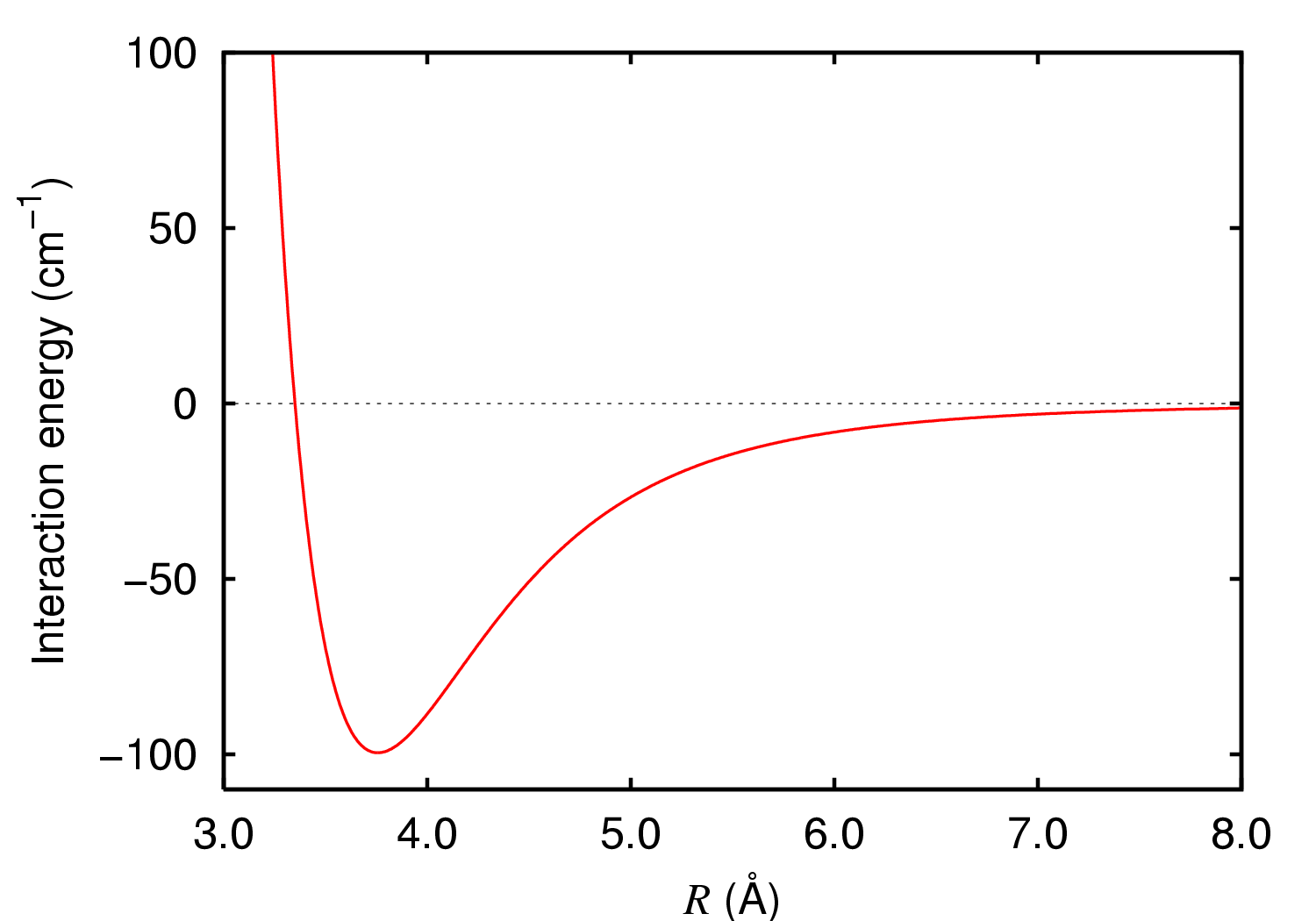 Two argon atoms are attracted together by
Two argon atoms are attracted together by
molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
containing two argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
s. Normally, this is only very weakly bound together by van der Waals force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and th ...
s (a van der Waals molecule
A Van der Waals molecule is a weakly bound complex of atoms or molecules held together by intermolecular attractions such as Van der Waals forces or by hydrogen bonds.
The name originated in the beginning of the 1970s when stable molecular clu ...
). However, in an excited state
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers to a ...
, or ionised state, the two atoms can be more tightly bound together, with significant spectral features. At cryogenic temperature
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
The 13th IIR International Congress of Refrigeration (held in Washington DC in 1971) endorsed a universal definition of “cryogenics” and “cr ...
s, argon gas can have a few percent of diargon molecules.
Theory
 Two argon atoms are attracted together by
Two argon atoms are attracted together by van der Waals force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and th ...
s when far from each other. When they are close, electrostatic force
Coulomb's inverse-square law, or simply Coulomb's law, is an experimental law of physics that quantifies the amount of force between two stationary, electrically charged particles. The electric force between charged bodies at rest is conventiona ...
s repel them. There is a balance point where the van der Waals force matches the opposing repelling force, where energy is at a minimum, represented as the trough in the graph of interaction energy versus distance. This distance is the ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. ...
of the unexcited argon dimer. In a vibrating molecule
A molecular vibration is a periodic motion of the atoms of a molecule relative to each other, such that the center of mass of the molecule remains unchanged. The typical vibrational frequencies range from less than 1013 Hz to approximately 1014 H ...
, the distance between the atoms bounces backwards and forwards from one side of the trough to the other. Faster vibrations will force the state up to higher levels in the energy trough. If the vibration is too much the molecule will break up. In a rotating molecule, the centrifugal force
In Newtonian mechanics, the centrifugal force is an inertial force (also called a "fictitious" or "pseudo" force) that appears to act on all objects when viewed in a rotating frame of reference. It is directed away from an axis which is paralle ...
drags the atoms apart, but can still be overcome by the attractive force. But if the rotation is too much the atoms break apart.
Properties
Theionisation energy
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule i ...
of the neutral molecule is 14.4558 eV (or 116593 cm−1).
The dissociation energy
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical s ...
of neutral Ar2 in the ground state is 98.7 cm−1 which is hundreds of times weaker than that of typical molecules. The dissociation energy of Ar2+ is 1.3144 eV or 10601 cm−1.
The Ar2 molecule can exist in a number of different vibration and rotational states. If the molecule is not rotating, there are eight different vibration states. But if the molecule spins fast, vibration is more likely to shake it apart, and at the 30th rotational level there are only two stable and one metastable state
In chemistry and physics, metastability denotes an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball i ...
of vibration. In combination, there are 170 different possibilities that are stable. In the metastable states, energy will be released if the molecule breaks apart into two separate atoms, but some extra energy is required to overcome the attraction between the atoms. Quantum tunneling
In physics, a quantum (plural quanta) is the minimum amount of any physical entity (physical property) involved in an interaction. The fundamental notion that a physical property can be "quantized" is referred to as "the hypothesis of quantizati ...
can result in the molecule breaking apart with no extra energy. However this takes time, which can vary from 10−11 seconds to several centuries. Molecules crashing into each other also results in breakup of the van der Waals molecules. At standard conditions this only takes about 100 picosecond
A picosecond (abbreviated as ps) is a unit of time in the International System of Units (SI) equal to 10−12 or (one trillionth) of a second. That is one trillionth, or one millionth of one millionth of a second, or 0.000 000 000 ...
s.
Excited states
Neutral
99.6% of theargon isotopes
Argon (18Ar) has 26 known isotopes, from 29Ar to 54Ar and 1 isomer (32mAr), of which three are stable (36Ar, 38Ar, and 40Ar). On the Earth, 40Ar makes up 99.6% of natural argon. The longest-lived radioactive isotopes are 39Ar with a half-life o ...
are 40Ar, so the spectrum observed in natural argon dimer will be due to the 40Ar40Ar isotopomer
Isotopomers or isotopic isomers are isomers with isotopic atoms, having the same number of each isotope of each element but differing in their positions. The result is that the molecules are either constitutional isomers or stereoisomers solely ...
. The following table lists different excited states.
Cation
References
Extra references
*Parson, J.M.; Siska, P.E.; Lee, Y.T., Intermolecular potentials from crossed-beam differential elastic scattering measurements. IV. Ar + Ar, J. Chem. Phys., 1972, 56, 1511. *LeRoy, R.J., Improved spectroscopic dissociation energy for ground-state Ar2, J. Chem. Phys., 1972, 57, 573. *Present, R.D., Collision diameter and well depth of the Ar-Ar interaction, J. Chem. Phys., 1973, 58, 2659. *Wilkinson, P.G., Absorption spectrum of argon in the 1070–1135 Å region, Can. J. Phys., 1968, 46, 315. *Tanaka, Y.; Yoshino, K., Absorption spectrum of the argon molecule in the vacuum-UV region, J. Chem. Phys., 1970, 53, 2012. *Colbourn, E.A.; Douglas, A.E., The spectrum and ground state potential curve of Ar2, J. Chem. Phys., 1976, 65, 1741. *Huffman, R.E.; Larrabee, J.C.; Tanaka, Y., Rare gas continuum light sources for photoelectric scanning in the vacuum ultraviolet, Appl. Opt., 1965, 4, 1581. *Wilkinson, P.G., The mechanism of the argon emission continuum in the vacuum ultraviolet. I, Can. J. Phys., 1967, 45, 1715. *Tanaka, Y., Continuous emission spectra of rare gases in the vacuum ultraviolet region, J. Opt. Soc. Am., 1955, 45, 710. *Strickler, T.D.; Arakawa, E.T., Optical emission from argon excited by alpha particles: quenching studies, J. Chem. Phys., 1964, 41, 1783. *Verkhovtseva, E.T.; Fogel, Ya.M.; Osyka, V.S., On the continuous spectra of inert gases in the vacuum-ultraviolet region obtained by means of a gas-jet source, Opt. Spectrosc. Engl. Transl., 1968, 25, 238, In original 440. *Hurst, G.S.; Bortner, T.E.; Strickler, T.D., Proton excitation of the argon atom, Phys. Rev., 1969, 178, 4. *Tanaka, Y.; Jursa, A.S.; LeBlanc, F.J., Continuous emission spectra of rare gases in the vacuum ultraviolet region. II. Neon and helium, J. Opt. Soc. Am., 1958, 48, 304. Michaelson, R.C.; Smith, A.L., Potential curves from emission continua. IV. The upper state of the vacuum uv contiua of Ar2, J. Chem. Phys., 1974, 61, 2566. ll data*Morgan, C.E.; Frommhold, L., Raman spectra of van der Waals dimers in argon, Phys. Rev. Lett., 1972, 29, 1053. *Frommhold, L.; Bain, R., Comments concerning the "Raman spectra of van der Waals dimers in argon", J. Chem. Phys., 1975, 63, 1700. *Cavallini, M.; Gallinaro, G.; Meneghetti, L.; Scoles, G.; Valbusa, U., Rainbow scattering and the intermolecular potential of argon, Chem. Phys. Lett., 1970, 7, 303. *Barker, J.A.; Fisher, R.A.; Watts, R.O., Liquid argon: Monte Carlo and molecular dynamics calculations, Mol. Phys., 1971, 21, 657. *Maitland, G.C.; Smith, E.B., The intermolecular pair potential of argon, Mol. Phys., 1971, 22, 861. *Present, R.D., Collision diameter and well depth of the Ar-Ar interaction, J. Chem. Phys., 1973, 58, 2659. *Photoionization of Ar2 at high resolution The Journal of Chemical Physics 76, 1263 (1982); https://doi.org/10.1063/1.443144 P. M. Dehmer **spectrum 800 to 850Å * Ab initio pair potential energy curve for the argon atom pair and thermophysical properties for the dilute argon gas. II. Thermophysical properties for low-density argon Eckhard Vogel, Benjamin Jäger, Robert Hellmann & Eckard Bich Pages 3335–3352 Published 07 Oct 2010 https://doi.org/10.1080/00268976.2010.507557 (will use the formula and draw graph) *Accurate ab initio potential for argon dimer including highly repulsive region Konrad Patkowski, Garold Murdachaew, Cheng-Ming Fou & Krzysztof Szalewicz Pages 2031–2045 Accepted 12 Sep 2004, Published online: 21 Feb 2007 https://doi.org/10.1080/00268970500130241 *The spectrum and ground state potential curve of Ar2 The Journal of Chemical Physics 65, 1741 (1976); https://doi.org/10.1063/1.433319 E. A. Colbourn and A. E. Douglas *The intermolecular pair potential of argon G.C. Maitland & E.B. Smith Pages 861–868 , Received 27 Oct 1971 https://doi.org/10.1080/00268977100103181 Molecular Physics An International Journal at the Interface Between Chemistry and Physics Volume 22, 1971 – Issue 5 *The Journal of Chemical Physics > Volume 61, Issue 8 Interpretation of Raman spectra of van der Waals dimers in argon The Journal of Chemical Physics 61, 2996 (1974); https://doi.org/10.1063/1.1682453 Lothar Frommhold *Volume 23, Issue 5, May 1980, Pages 499–502 Journal of Quantitative Spectroscopy and Radiative Transfer ''On the Hulburt-Hirschfelder potential function for the Ar2 molecule'' Swadesh Kumar Ghoshal; Sankar Sengupta https://doi.org/10.1016/0022-4073(80)90052-7 *Volume 71, Issue 4 > 10.1063/1.438529 Emission spectrum of rare gas dimers in the vacuum UV region. II. Rotational analysis of band system I of Ar2 The Journal of Chemical Physics 71, 1780 (1979); https://doi.org/10.1063/1.438529 D. E. Freeman, K. Yoshino, and Y. Tanakam (1073.5–1081.5 Å) *Imaging of the Structure of the Argon and Neon Dimer, Trimer, and Tetramer B. Ulrich, A. Vredenborg, A. Malakzadeh†, L. Ph. H. Schmidt, T. Havermeier, M. Meckel†, K. Cole, M. Smolarski‡, Z. Chang, T. Jahnke, and R. Dörner J. Phys. Chem. A, 2011, 115 (25), pp 6936–6941 DOI: 10.1021/jp1121245 http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.661.7525&rep=rep1&type=pdf *''Experimental evidence for two decay channels in electron impact ionization and fragmentation of argon dimer'' Elias Jabbour Al Maalouf1, Xueguang Ren2,3, Alexander Dorn2 and Stephan Denifl Journal of Physics: Conference Series, Volume 635, Lepton – Molecule and Small Cluster http://iopscience.iop.org/article/10.1088/1742-6596/635/7/072062/pdf *''Raman studies of argon dimers in a supersonic expansion. I. Spectroscopy'' H. P. Godfried and Isaac F. Silvera Phys. Rev. A 27, 3008 – Published 1 June 1983https://pure.uva.nl/ws/files/2168366/46711_214418y.pdf https://doi.org/10.1103/PhysRevA.27.3008 *''Observation of dissociative recombination of Ne+2 and Ar+2 directly to the ground state of the product atoms'' G. B. Ramos, M. Schlamkowitz, J. Sheldon, K. A. Hardy, and J. R. Peterson Phys. Rev. A 51, 2945 – Published 1 April 1995 https://doi.org/10.1103/PhysRevA.51.2945 *''Dissociative recombination studies of Ar+2 by time-of-flight spectroscopy'' G. B. Ramos, M. Schlamkowitz, J. Sheldon, K. Hardy, and J. R. Peterson Phys. Rev. A 52, 4556 – Published 1 December 1995 https://doi.org/10.1103/PhysRevA.52.4556 {{Noble gas compounds Argon compounds Allotropes Van der Waals molecules