|
Oxazepine
Oxazepines are a family of unsaturated heterocycles containing seven atoms, with a nitrogen replacing a carbon at one position and with an oxygen replacing a carbon at one position. See also * Azepine * Benzazepines * Diazepine * Oxepin * Borepin * CR gas CR gas or dibenzoxazepine (chemical name dibenz 'b'',''f''1,4]oxazepine, is an incapacitating agent and a lachrymatory agent. CR was developed by the British Ministry of Defence as a riot control agent in the late 1950s and early 1960s. A report ... (dibenzoxazepine) References Oxazepines, [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CR Gas
CR gas or dibenzoxazepine (chemical name dibenz 'b'',''f''1,4]oxazepine, is an incapacitating agent and a lachrymatory agent. CR was developed by the British Ministry of Defence as a riot control agent in the late 1950s and early 1960s. A report from the Porton Down laboratories described exposure as "like being thrown blindfolded into a bed of stinging nettles", and it earned the nickname "firegas". In its effects, the CR gas is very similar (but twice as potent) to o-chlorobenzylidene malononitrile CS gas, even though there is little structural resemblance between the two. For example, 2 mg of dry CR cause skin redness in 10 min, 5 mg cause burning and erythremia, 20 mg- strong pain. Water usually amplifies the pain effect of CR on skin. CR aerosols cause irritation as 0.0002 mg/L, which becomes intolerable at 0.003 mg/L. The lethal dose of CR through air inhalation is LD50 = 350 mg·min/L. Physical properties and deployment CR is a pale yellow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different chemical element, elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids ( DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere. Many indus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Description The combining capacity, or affinity of an ...—its atom making four electrons available to form covalent bond, covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes up only about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, Carbon-12, C and Carbon-13, C being stable, while Carbon-14, C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the Timeline of chemical element discoveries#Ancient discoveries, few elements known since antiquity. Carbon is the 15th Abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the Abundance of the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats, as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michigan State University
Michigan State University (Michigan State, MSU) is a public university, public Land-grant university, land-grant research university in East Lansing, Michigan. It was founded in 1855 as the Agricultural College of the State of Michigan, the first of its kind in the United States. It is considered a Public Ivy, or a public institution which offers an academic experience similar to that of an Ivy League university. After the introduction of the Morrill Land-Grant Acts, Morrill Act in 1862, the state designated the college a land-grant institution in 1863, making it the first of the land-grant colleges in the United States. The college became coeducational in 1870. In 1955, the state officially made the college a university, and the current name, Michigan State University, was adopted in 1964. Today, Michigan State has the largest undergraduate enrollment among Michigan's colleges and universities and approximately 634,300 living alums worldwide. The university is a member of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azepine
Azepines are unsaturated heterocycles of seven atoms, with a nitrogen replacing a carbon at one position. See also * Azepane * Benzazepines * Diazepine * Oxepin * Borepin Borepins are a class of boron-containing heterocycles used in main group chemistry. They consist of a seven-membered unsaturated ring with a tricoordinate boron in it. Simple borepins are analogues of cycloheptatriene, which is a seven-membered ... Azepines {{Heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzazepines
Benzazepines are heterocyclic chemical compounds consisting of a benzene ring fused to an azepine ring. Examples include: File:Benazepril structure.svg, Benazepril File:Fenoldopam.svg, Fenoldopam File:GSK-189,254.svg, GSK-189,254 File:Ivabradine 2.svg, Ivabradine File:Semagacestat structure.svg, Semagacestat File:Varenicline.svg, Varenicline See also * Benzodiazepine Benzodiazepines (BZD, BDZ, BZs), sometimes called "benzos", are a class of depressant drugs whose core chemical structure is the fusion of a benzene ring and a diazepine ring. They are prescribed to treat conditions such as anxiety disorders, ... * Dibenzazepine References {{Heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazepine
Diazepine is a seven-membered heterocyclic compound with two nitrogen atoms (e.g., in ring positions 1 and 2). Types include: * 1,2-diazepine * 1,3-diazepine * 1,4-diazepine ** Benzodiazepines Benzodiazepines (BZD, BDZ, BZs), sometimes called "benzos", are a class of depressant drugs whose core chemical structure is the fusion of a benzene ring and a diazepine ring. They are prescribed to treat conditions such as anxiety disorders, i ..., a class of commonly used medications whose structure contains a 1,4-diazepine ring {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxepin
Oxepin is an oxygen-containing heterocycle consisting of a seven-membered ring with three double bonds. The parent C6H6O exists as an equilibrium mixture with benzene oxide. The oxepin–benzene oxide equilibrium is affected by the ring substituents. A related dimethyl derivative exists mainly as the oxepin isomer, an orange liquid. Oxepin is an intermediate in the oxidation of benzene by the cytochrome P450 (CYP). Other arene oxide In chemistry, an arene oxide is an epoxide of an arene. Two important families of arene oxides are benzene oxides and naphthalene oxides as these are intermediates in the oxidative degradation of benzene and naphthalene, two common pollutants. ...s are metabolites of the parent arene. References {{Reflist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
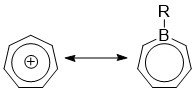
Borepin
Borepins are a class of boron-containing heterocycles used in main group chemistry. They consist of a seven-membered unsaturated ring with a tricoordinate boron in it. Simple borepins are analogues of cycloheptatriene, which is a seven-membered ring containing three carbon-carbon double bonds, each of which contributes 2π electrons for a total of 6π electrons. Unlike other seven-membered systems such as silepins and phosphepins, boron has a vacant p-orbital that can interact with the π and π* orbitals of the cycloheptatriene. This leads to an isoelectronic state akin to that of the tropylium cation, aromatizing the borepin while also allowing it to act as a Lewis acid. The aromaticity of borepin is relatively weak compared to traditional aromatics such as benzene or even cycloheptatriene, which has led to the synthesis of many fused, π-conjugated borepin systems over the years. Simple and complex borepins have been extensively studied more recently due to their high fluor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |