Borepin on:
[Wikipedia]
[Google]
[Amazon]
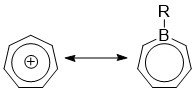 Borepins are a class of boron-containing heterocycles used in
Borepins are a class of boron-containing heterocycles used in
 The first synthesis of a stable borepin was reported in 1960 by van Tamelen, Brieger, and Untch. The synthesis began with a
The first synthesis of a stable borepin was reported in 1960 by van Tamelen, Brieger, and Untch. The synthesis began with a
 Eisch and Galle isolated the first non-fused borepin in 1975. The heptaphenyl borabicycloheptadiene on the left went through a suprafacial sigmatropic rearrangement, leading to the intermediate in the middle. This intermediate subsequently underwent
Eisch and Galle isolated the first non-fused borepin in 1975. The heptaphenyl borabicycloheptadiene on the left went through a suprafacial sigmatropic rearrangement, leading to the intermediate in the middle. This intermediate subsequently underwent 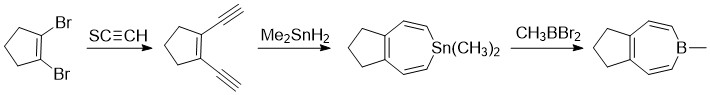 More recently a method for a minimally substituted borepin was developed by Ashe and Drone. They proceeded from 1,2-dibromocyclopentene and performed a van der Kerk method for boron heterocycle preparation. Next, they initiated a
More recently a method for a minimally substituted borepin was developed by Ashe and Drone. They proceeded from 1,2-dibromocyclopentene and performed a van der Kerk method for boron heterocycle preparation. Next, they initiated a

 Chemists like Ashe were able to utilize this knowledge in the ‘90s to functionalize borepins as a compound, leading to the formation of many Lewis acid-base adducts. The most common borepin precursor used by chemists is a borepin-halide complex as halides are a good
Chemists like Ashe were able to utilize this knowledge in the ‘90s to functionalize borepins as a compound, leading to the formation of many Lewis acid-base adducts. The most common borepin precursor used by chemists is a borepin-halide complex as halides are a good 
 Most recently, in 2022 Gilliard et. al. were able to apply similar principles from their cationic borepins to form and characterize the first instance of isolated borepin radicals. These radicals were also capable of being reduced to the first instance of a borepin
Most recently, in 2022 Gilliard et. al. were able to apply similar principles from their cationic borepins to form and characterize the first instance of isolated borepin radicals. These radicals were also capable of being reduced to the first instance of a borepin 
 An interesting complication that arises with fusion of the phenyl rings is their positioning. When synthesizing dibenzo ,forepins (b is the carbon next to the boron atom) they are perfectly aligned for conjugation of the borocycloheptatriene ring. However, if the phenyls are positioned in a ,efashion (see below) then the resulting compound is less stable than dizbenzo ,forepins by around 34 kcal/mol, quite a large energy difference.
An interesting complication that arises with fusion of the phenyl rings is their positioning. When synthesizing dibenzo ,forepins (b is the carbon next to the boron atom) they are perfectly aligned for conjugation of the borocycloheptatriene ring. However, if the phenyls are positioned in a ,efashion (see below) then the resulting compound is less stable than dizbenzo ,forepins by around 34 kcal/mol, quite a large energy difference.
 These results explained by Schulman and Disch have been applied many times over to modify borepin frameworks. Some common examples include increasing the number of rings--making boron-doped polycyclic-aromatic hydrocarbons (PAHs), adding additional R groups to the framework such as
These results explained by Schulman and Disch have been applied many times over to modify borepin frameworks. Some common examples include increasing the number of rings--making boron-doped polycyclic-aromatic hydrocarbons (PAHs), adding additional R groups to the framework such as  The rapid development of borepin stabilization and functionalization since the 2000s has catapulted studies of complex and versatile molecules. Like many other main group compounds, borepins have been in the field since the mid-late 1900s yet lay dormant until more modern methods could utilize them.
The rapid development of borepin stabilization and functionalization since the 2000s has catapulted studies of complex and versatile molecules. Like many other main group compounds, borepins have been in the field since the mid-late 1900s yet lay dormant until more modern methods could utilize them.
 In contrast to that example, upon addition of cyanide to one of their borepin analogues to tetrathienoanthracence, Adachi and Ohshita saw a loss of fluorescence. However, upon cooling, there was a noticeable
In contrast to that example, upon addition of cyanide to one of their borepin analogues to tetrathienoanthracence, Adachi and Ohshita saw a loss of fluorescence. However, upon cooling, there was a noticeable 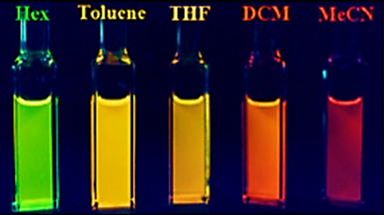
 Borepins are a class of boron-containing heterocycles used in
Borepins are a class of boron-containing heterocycles used in main group
In chemistry and atomic physics, the main group is the group of elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, and fluorine as arra ...
chemistry. They consist of a seven-membered unsaturated ring with a tricoordinate boron in it. Simple borepins are analogues of cycloheptatriene
Cycloheptatriene (CHT) is an organic compound with the formula C7H8. It is a closed ring of seven carbon atoms joined by three double bonds (as the name implies) and four single bonds. This colourless liquid has been of recurring theoretical inter ...
, which is a seven-membered ring containing three carbon-carbon double bonds, each of which contributes 2π electrons for a total of 6π electrons. Unlike other seven-membered systems such as silepins and phosphepins, boron has a vacant p-orbital that can interact with the π and π* orbitals of the cycloheptatriene. This leads to an isoelectronic state akin to that of the tropylium cation
In organic chemistry, the tropylium ion or cycloheptatrienyl cation is an aromatic species with a formula of 7H7sup>+. Its name derives from the molecule tropine from which cycloheptatriene (tropylidene) was first synthesized in 1881. Salts of ...
, aromatizing the borepin while also allowing it to act as a Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
. The aromaticity
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturate ...
of borepin is relatively weak compared to traditional aromatics such as benzene or even cycloheptatriene, which has led to the synthesis of many fused, π-conjugated borepin systems over the years. Simple and complex borepins have been extensively studied more recently due to their high fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, tha ...
and potential applications in technologies like organic light-emitting diodes (OLEDs
An organic light-emitting diode (OLED or organic LED), also known as organic electroluminescent (organic EL) diode, is a light-emitting diode (LED) in which the emission (electromagnetic radiation), emissive electroluminescence, electroluminesc ...
) and photovoltaic cells
A solar cell, or photovoltaic cell, is an electronic device that converts the energy of light directly into electricity by the photovoltaic effect, which is a physical and chemical phenomenon.
.
Synthesis
First reported synthetic method
 The first synthesis of a stable borepin was reported in 1960 by van Tamelen, Brieger, and Untch. The synthesis began with a
The first synthesis of a stable borepin was reported in 1960 by van Tamelen, Brieger, and Untch. The synthesis began with a lithiation
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
of o,o’-dibromobibenzyl. Next it was reacted with tributyl borate to yield a fused borinic acid ring. This product was reacted with n-bromosuccinimide
''N''-Bromosuccinimide or NBS is a chemical reagent used in radical substitution, electrophilic addition, and electrophilic substitution reactions in organic chemistry. NBS can be a convenient source of Br•, the bromine radical.
Preparation
...
(NBS) to yield a bromo-substituted product. Finally, they performed a dehydrohalogenation
In chemistry, dehydrohalogenation is an elimination reaction which removes a hydrogen halide from a substrate. The reaction is usually associated with the synthesis of alkenes, but it has wider applications.
Dehydrohalogenation from alkyl halid ...
to yield the borepin ring system seen above. A method similar to this involving a tin-boron exchange is commonly used in modern synthesis of fused borepin systems.
Synthetic developments
 Eisch and Galle isolated the first non-fused borepin in 1975. The heptaphenyl borabicycloheptadiene on the left went through a suprafacial sigmatropic rearrangement, leading to the intermediate in the middle. This intermediate subsequently underwent
Eisch and Galle isolated the first non-fused borepin in 1975. The heptaphenyl borabicycloheptadiene on the left went through a suprafacial sigmatropic rearrangement, leading to the intermediate in the middle. This intermediate subsequently underwent disrotatory
An electrocyclic reaction can either be classified as conrotatory or disrotatory based on the rotation at each end of the molecule. In conrotatory mode, both atomic orbitals of the end groups turn in the same direction (such as both atomic orbital ...
ring opening to yield heptaphenylborepin on the right. The isolated borepin is kinetically stabilized by the bulky phenyl groups bound to all seven positions on the ring, protecting it from reactions with moisture in the air. However, like most borepins, this compound reacted with oxygen, turning from fluorescent green to purple.
 More recently a method for a minimally substituted borepin was developed by Ashe and Drone. They proceeded from 1,2-dibromocyclopentene and performed a van der Kerk method for boron heterocycle preparation. Next, they initiated a
More recently a method for a minimally substituted borepin was developed by Ashe and Drone. They proceeded from 1,2-dibromocyclopentene and performed a van der Kerk method for boron heterocycle preparation. Next, they initiated a ring closure
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where ...
to form a 7-membered tin complex. Finally, they completed a tin-boron exchange reaction to afford the bicyclic borepin on the right.
Previous synthetic methods yielded heavily substituted and bulky borepin compounds such as heptaphenyl borepin. These routes, while generating very stable complexes, made it difficult to analyze the properties of the borepin ring. Minimal substitution allowed scientists like Ashe to confirm the presence of aromaticity and ring currents
Ring may refer to:
* Ring (jewellery), a round band, usually made of metal, worn as ornamental jewelry
* To make a sound with a bell, and the sound made by a bell
:(hence) to initiate a telephone connection
Arts, entertainment and media Film and ...
within the borepin system.
As more modern methods appeared, the tin-boron exchange reaction has become more commonly used as tin can act as a placeholder in the seven-membered ring, reacting with boryl halides quite easily.
Isomerization
As a final note, in 2018 theBraunschweig
Braunschweig () or Brunswick ( , from Low German ''Brunswiek'' , Braunschweig dialect: ''Bronswiek'') is a city in Lower Saxony, Germany, north of the Harz Mountains at the farthest navigable point of the river Oker, which connects it to the Nor ...
group synthesized a valence isomer In organic chemistry, two molecules are valence isomers when they are constitutional isomers that can interconvert through pericyclic reactions.
Benzene
There are many valence isomers one can draw for the C6H6 formula benzene. Some were originally ...
of borepin, shown below. This bicyclic, boron-containing heterocycle can be interconverted to its borepin isomer using pericyclic
In organic chemistry, a pericyclic reaction is the type of organic reaction wherein the transition state of the molecule has a cyclic geometry, the reaction progresses in a concerted fashion, and the bond orbitals involved in the reaction overlap ...
, photochemical
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 nm), visible light (400–7 ...
reactions.

Reactivity
While direct functionalization of the boron atom is possible due to its vacant p-orbital, most simple borepins are simply too reactive with air and moisture to be isolated. Therefore, borepins have been stabilized by two general methods: bulky, kinetically stabilizing ligands bound to the boron center and additional aromatic π-systems that can donate electron density into the empty boron p-orbital.Lewis acid-base adducts
Borepins are of interest due to their Lewis acidity.Density Functional Theory
Density-functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ground state) of many-body ...
(DFT) calculations have shown that the HOMO
''Homo'' () is the genus that emerged in the (otherwise extinct) genus ''Australopithecus'' that encompasses the extant species ''Homo sapiens'' ( modern humans), plus several extinct species classified as either ancestral to or closely relate ...
of borepin lies mostly with the carbon moieties of the seven-membered ring, while the LUMO
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontie ...
is centered around the boron atom. An example of the HOMO/LUMO distribution can be seen in the figure below.
 Chemists like Ashe were able to utilize this knowledge in the ‘90s to functionalize borepins as a compound, leading to the formation of many Lewis acid-base adducts. The most common borepin precursor used by chemists is a borepin-halide complex as halides are a good
Chemists like Ashe were able to utilize this knowledge in the ‘90s to functionalize borepins as a compound, leading to the formation of many Lewis acid-base adducts. The most common borepin precursor used by chemists is a borepin-halide complex as halides are a good leaving group In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited t ...
. The borepin-hydride complex has not been able to be isolated due to its instability, whereas the boron-doped spirocyle on the right side satisfies boron’s octet, forming a zwitterion
In chemistry, a zwitterion ( ; ), also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively- and negatively-charged functional groups.
: With amino acids, for example, in solution a chemical equilibrium wil ...
between boron and nitrogen.

Borepin cations, anions, and radicals
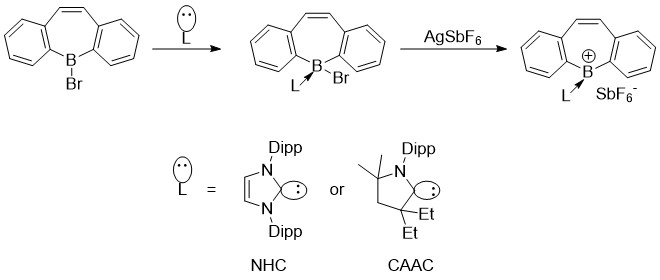
Using the concept of zwitterions, Gilliard et. al. was recently able to synthesize and characterize acationic
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
borepin state using N-heterocyclic carbenes
A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for ex ...
(NHCs) and cyclic(alkyl)(amino)carbenes (CAACS). Due to the dative donation of NHCs and CAACs, boron has only two covalent bonds, giving it a formal positive charge.
 Most recently, in 2022 Gilliard et. al. were able to apply similar principles from their cationic borepins to form and characterize the first instance of isolated borepin radicals. These radicals were also capable of being reduced to the first instance of a borepin
Most recently, in 2022 Gilliard et. al. were able to apply similar principles from their cationic borepins to form and characterize the first instance of isolated borepin radicals. These radicals were also capable of being reduced to the first instance of a borepin anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
where there is multiple bonding between a boron-carbon center. The generation of the radical comes from the strong π-accepting ability of the carbene carbon. The electron density shared with the boron center back bonds slightly with the carbon atom, leading to the single-electron radical species.

Framework manipulation
Spectroscopic data, DFT calculations, and thermochemical data have shown that borepin is weakly aromatic when compared to the tropylium cation. This reduction in aromaticity leads to increased reactivity and instability at the boron center as there is less electron density being donated to boron’s p-orbital. As a result, chemists sought ways to increase the aromatic character of borepins. The tried-and-true method by which chemists stabilize borepins is phenyl-borepin ring fusion (annulation
In organic chemistry annulation (from the Latin ''anellus'' for "little ring"; occasionally annelation) is a chemical reaction in which a new ring is constructed on a molecule.
:
Examples are the Robinson annulation, Danheiser annulation and cert ...
). The addition of two fused phenyl rings increases the 6π borepin system to a 14π fused system.
 An interesting complication that arises with fusion of the phenyl rings is their positioning. When synthesizing dibenzo ,forepins (b is the carbon next to the boron atom) they are perfectly aligned for conjugation of the borocycloheptatriene ring. However, if the phenyls are positioned in a ,efashion (see below) then the resulting compound is less stable than dizbenzo ,forepins by around 34 kcal/mol, quite a large energy difference.
An interesting complication that arises with fusion of the phenyl rings is their positioning. When synthesizing dibenzo ,forepins (b is the carbon next to the boron atom) they are perfectly aligned for conjugation of the borocycloheptatriene ring. However, if the phenyls are positioned in a ,efashion (see below) then the resulting compound is less stable than dizbenzo ,forepins by around 34 kcal/mol, quite a large energy difference.
 These results explained by Schulman and Disch have been applied many times over to modify borepin frameworks. Some common examples include increasing the number of rings--making boron-doped polycyclic-aromatic hydrocarbons (PAHs), adding additional R groups to the framework such as
These results explained by Schulman and Disch have been applied many times over to modify borepin frameworks. Some common examples include increasing the number of rings--making boron-doped polycyclic-aromatic hydrocarbons (PAHs), adding additional R groups to the framework such as alkynes
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
and long-chain alkanes, and even introducing electron-rich heteroatoms such as nitrogen or sulfur in order to further stabilize the borepins. Some examples of these compounds can be seen in the image below:
 The rapid development of borepin stabilization and functionalization since the 2000s has catapulted studies of complex and versatile molecules. Like many other main group compounds, borepins have been in the field since the mid-late 1900s yet lay dormant until more modern methods could utilize them.
The rapid development of borepin stabilization and functionalization since the 2000s has catapulted studies of complex and versatile molecules. Like many other main group compounds, borepins have been in the field since the mid-late 1900s yet lay dormant until more modern methods could utilize them.
Photophysical properties
Fluorescence/phosphorescence
The first reports offluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, tha ...
in a borepin was published in 1975 by Eisch and Galle and described how heptaphenylborepin was fluorescent green when probed. Little photophysical phenomena were recorded for many years, until Piers’s group published the first example of a blue-fluorescent borepin species in 2009. They discovered that by expanding the π-system (i.e. adding more fused phenyl rings) they could dramatically shift the wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, tro ...
of their compounds from around 250 nanometers (nm) to upwards of 450 nm. The rationale behind this shift is that the presence of boron in the aromatic system decreases the energy gap between the HOMO and LUMO, resulting in changing absorptions and greater intensity of fluorescence. Similar results were reported by Caruso, Tovar, and Siegler in 2010 when they ran borepins through electrochemical redox reactions
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
and by Messersmith, Siegler, and Tovar in 2016 when testing the effects of variable aromaticity of borepins.
The initial excitement behind these results was the potential for use in electronic materials such as organic light-emitting diodes (OLEDs
An organic light-emitting diode (OLED or organic LED), also known as organic electroluminescent (organic EL) diode, is a light-emitting diode (LED) in which the emission (electromagnetic radiation), emissive electroluminescence, electroluminesc ...
). If the fluorescence “switch” could be controlled, in addition to having stable borepin complexes, then it would be relatively easy and cheap to achieve bright fluorescent lights, potentially of any color.
Another potential of redox chemistry is the use of boron-containing polycyclic aromatic hydrocarbons as semiconductors
A semiconductor is a material which has an electrical resistivity and conductivity, electrical conductivity value falling between that of a electrical conductor, conductor, such as copper, and an insulator (electricity), insulator, such as glas ...
. Because of borepins’ low-lying LUMO, it can act as an electron acceptor to participate in electron transport. The Wagner group as well as Toscano and co-workers showed computationally and experimentally the potential applications for these complexes.
On another note, scientists have sought to utilize borepins as potential anion sensors. In the past, tri-coordinate boranes have been used to detect anions like fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typ ...
, cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of ...
, and even ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
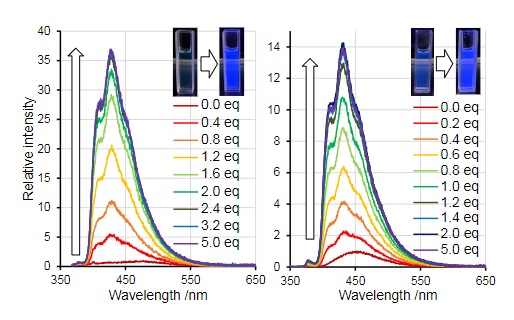
. Scientists like Adachi and Ohshita have demonstrated that upon coordination of fluoride (F-) fluorescence increases by many magnitudes.
 In contrast to that example, upon addition of cyanide to one of their borepin analogues to tetrathienoanthracence, Adachi and Ohshita saw a loss of fluorescence. However, upon cooling, there was a noticeable
In contrast to that example, upon addition of cyanide to one of their borepin analogues to tetrathienoanthracence, Adachi and Ohshita saw a loss of fluorescence. However, upon cooling, there was a noticeable phosphorescence
Phosphorescence is a type of photoluminescence related to fluorescence. When exposed to light (radiation) of a shorter wavelength, a phosphorescent substance will glow, absorbing the light and reemitting it at a longer wavelength. Unlike fluo ...
in solution.
Fluorescence is not only limited to outside coordination. Upon insertion of nitrogen into the borepin ring, Li et. al. were able to observe solvatochromic In chemistry, solvatochromism is the phenomenon observed when the colour due to a solute is different when that solute is dissolved in different solvents.
The solvatochromic effect is the way the spectrum of a substance (the solute) varies when th ...
effects. Upon addition of the borepin to hexanes
Hexane () is an organic compound, a straight-chain alkane with six carbon atoms and has the molecular formula C6H14.
It is a colorless liquid, odorless when pure, and with boiling points approximately . It is widely used as a cheap, relatively ...
, toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) at ...
, tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is ma ...
(THF), dichloromethane
Dichloromethane (DCM or methylene chloride, methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with ...
(DCM) and acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not clas ...
(MeCN), rather drastic changes in color were observed.

References
{{reflist Boron heterocycles Seven-membered rings