|
Organosilicon Compound
Organosilicon compounds are organometallic compounds containing carbon–silicon bonds. Organosilicon chemistry is the corresponding science of their preparation and properties. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an ''inorganic'' compound. History In 1846 Von Ebelman's had synthesized Tetraethyl orthosilicate (Si(OC2H5)4). In 1863 Friedel and Crafts managed to make the first organosilieon compound with C-Si bonds which gone byound the syntheses of orthosilicic acid esters. The same year they also described a «polysilicic acid ether» in the preparation of ethyl- and methyl-o-silicic acid. The early extensive research in the field of organosilicon compounds was pioneerd in the beginning of 20th century by Frederic Kipping. He also had coined the term «silicone» (akin to ketones) in relation to these materials in 1904. In recognition of Kipping's achiev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Herbicide
Herbicides (, ), also commonly known as weedkillers, are substances used to control undesired plants, also known as weeds.EPA. February 201Pesticides Industry. Sales and Usage 2006 and 2007: Market Estimates. Summary in press releasMain page for EPA reports on pesticide use ihere Selective herbicides control specific weed species, while leaving the desired crop relatively unharmed, while non-selective herbicides (sometimes called total weedkillers in commercial products) can be used to clear waste ground, industrial and construction sites, railways and railway embankments as they kill all plant material with which they come into contact. Apart from selective/non-selective, other important distinctions include ''persistence'' (also known as ''residual action'': how long the product stays in place and remains active), ''means of uptake'' (whether it is absorbed by above-ground foliage only, through the roots, or by other means), and ''mechanism of action'' (how it works). Histo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Dissociation Energy
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical species. The enthalpy change is temperature-dependent, and the bond-dissociation energy is often defined to be the enthalpy change of the homolysis at 0 K (absolute zero), although the enthalpy change at 298 K (standard conditions) is also a frequently encountered parameter. As a typical example, the bond-dissociation energy for one of the C−H bonds in ethane () is defined as the standard enthalpy change of the process : , : ''DH''°298() = Δ''H°'' = 101.1(4) kcal/mol = 423.0 ± 1.7 kJ/mol = 4.40(2) eV (per bond). To convert a molar BDE to the energy needed to dissociate the bond ''per molecule'', the conversion factor 23.060 kcal/mol (96.485 kJ/mol) for each eV can be used. A variety of experim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Picometer
The picometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: pm) or picometer ( American spelling) is a unit of length in the International System of Units (SI), equal to , or one trillionth of a metre, which is the SI base unit of length. The picometre is one thousand femtometres, one thousandth of a nanometre ( nm), one millionth of a micrometre (also known as a micron), one billionth of a millimetre, and one trillionth of a metre. The symbol μμ was once used for it. It is also one hundredth of an ångström, an internationally known (but non-SI) unit of length. Use The picometre's length is of an order so small that its application is almost entirely confined to particle physics, quantum physics, chemistry and acoustics. Atoms are between 62 and 520 pm in diameter, and the typical length of a carbon–carbon single bond is 154 pm. Smaller units still may be used to describe smaller particles (some of whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedral Molecular Geometry
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1(−) = 109.4712206...° ≈ 109.5° when all four substituents are the same, as in methane () as well as its heavier analogues. Methane and other perfectly symmetrical tetrahedral molecules belong to point group Td, but most tetrahedral molecules have lower symmetry. Tetrahedral molecules can be chiral. Tetrahedral bond angle The bond angle for a symmetric tetrahedral molecule such as CH4 may be calculated using the dot product of two vectors. As shown in the diagram, the molecule can be inscribed in a cube with the tetravalent atom (e.g. carbon) at the cube centre which is the origin of coordinates, O. The four monovalent atoms (e.g. hydrogens) are at four corners of the cube (A, B, C, D) chosen so that no two atoms are at adjacent corners linked by only one cube edge. If the edge length of the cube ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Description The combining capacity, or affinity of an atom of a given element is determined by the number of hydrogen atoms that it combines with. In methane, carbon has a valence of 4; in ammonia, nitrogen has a valence of 3; in water, oxygen has a valence of 2; and in hydrogen chloride, chlorine has a valence of 1. Chlorine, as it has a valence of one, can be substituted for hydrogen. Phosphorus has a valence of 5 in phosphorus pentachloride, . Valence diagrams of a compound represent the connectivity of the elements, with lines drawn between two elements, sometimes called bonds, representing a saturated valency for each element. The two tables below show some examples of different compounds, their valence diagrams, and the valences for each element of the compound. Modern definitions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Insecticide
Insecticides are substances used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. Insecticides are used in agriculture, medicine, industry and by consumers. Insecticides are claimed to be a major factor behind the increase in the 20th-century's agricultural productivity. Nearly all insecticides have the potential to significantly alter ecosystems; many are toxic to humans and/or animals; some become concentrated as they spread along the food chain. Insecticides can be classified into two major groups: systemic insecticides, which have residual or long term activity; and contact insecticides, which have no residual activity. The mode of action describes how the pesticide kills or inactivates a pest. It provides another way of classifying insecticides. Mode of action can be important in understanding whether an insecticide will be toxic to unrelated species, such as fish, birds and mammals. Insecticides may be repe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrethroid
A pyrethroid is an organic compound similar to the natural pyrethrins, which are produced by the flowers of pyrethrums (''Chrysanthemum cinerariaefolium'' and '' C. coccineum''). Pyrethroids are used as commercial and household insecticides. In household concentrations pyrethroids are generally harmless to humans. However, pyrethroids are toxic to insects such as bees, dragonflies, mayflies, gadflies, and some other invertebrates, including those that constitute the base of aquatic and terrestrial food webs. Pyrethroids are toxic to aquatic organisms, especially fish.Pyrethroids fact sheet from the Illinois Department of Public Health. They have been shown to be an effective control measure for malaria outbreaks, through indoor applications. Mode of action Pyrethroid ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silafluofen
Silafluofen is a fluorinated organosilicon pyrethroid insecticide Insecticides are substances used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. Insecticides are used in agriculture, medicine, industry and by consumers. Insecticides are claimed to b .... Silafluofen is used agriculturally against soil-borne insects such as termites, and as a wood preservative. It is registered in Asia (India, Japan, Taiwan, Vietnam) since at least 1995 for crops such as drupes, tea and rice, but has not been notified or authorised in the European Union for example. References Pyrethroids Organofluorides Organosilicon compounds Diphenyl ethers {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diatom
A diatom (New Latin, Neo-Latin ''diatoma''), "a cutting through, a severance", from el, διάτομος, diátomos, "cut in half, divided equally" from el, διατέμνω, diatémno, "to cut in twain". is any member of a large group comprising several Genus, genera of algae, specifically microalgae, found in the oceans, waterways and soils of the world. Living diatoms make up a significant portion of the Earth's Biomass (ecology), biomass: they generate about 20 to 50 percent of the oxygen produced on the planet each year, take in over 6.7 billion metric tons of silicon each year from the waters in which they live, and constitute nearly half of the organic material found in the oceans. The Protist shell, shells of dead diatoms can reach as much as a half-mile (800 m) deep on the ocean floor, and the entire Amazon basin is fertilized annually by 27 million tons of diatom shell dust transported by transatlantic winds from the African Sahara, much of it from the Bodélé Dep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
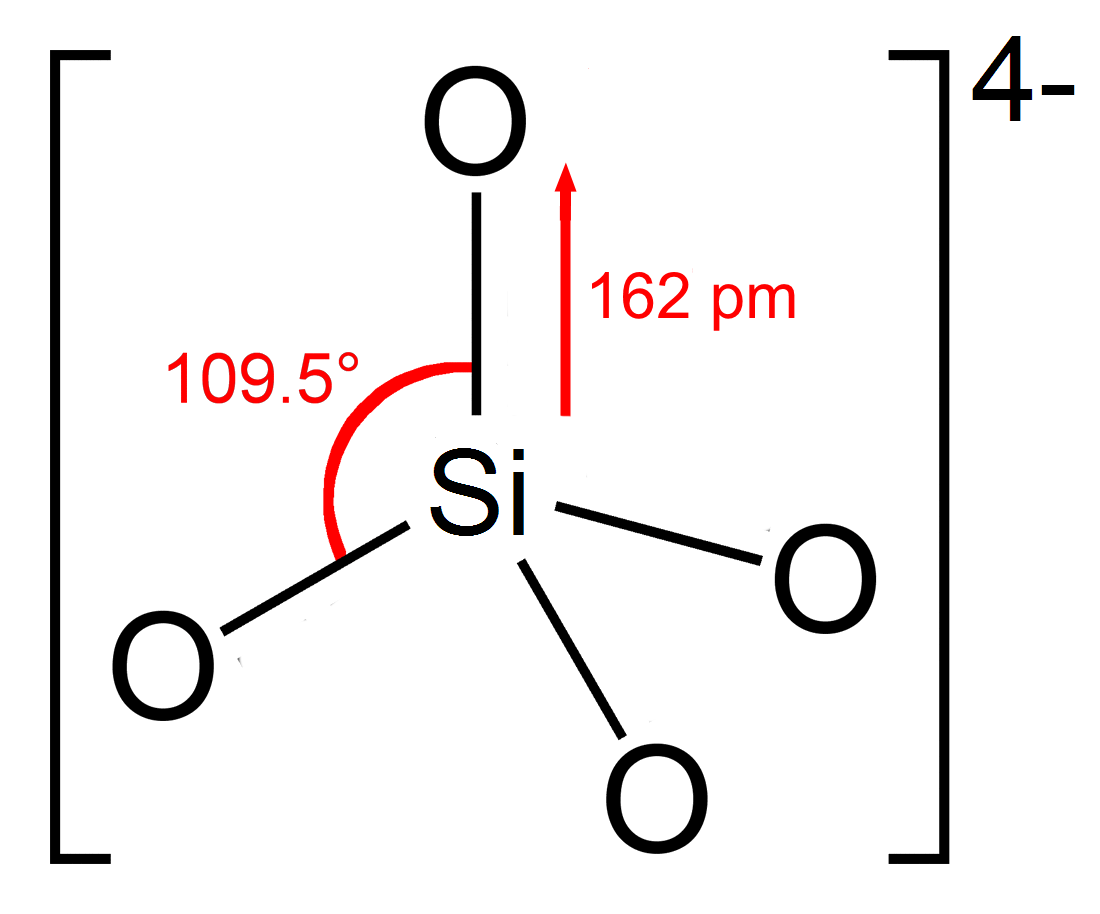
Silicate
In chemistry, a silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used for any salt of such anions, such as sodium metasilicate; or any ester containing the corresponding chemical group, such as tetramethyl orthosilicate. The name "silicate" is sometimes extended to any anions containing silicon, even if they do not fit the general formula or contain other atoms besides oxygen; such as hexafluorosilicate .Most commonly, silicates are encountered as silicate minerals. For diverse manufacturing, technological, and artistic needs, silicates are versatile materials, both natural (such as granite, gravel, and garnet) and artificial (such as Portland cement, ceramics, glass, and waterglass). Structural principles In all silicates, silicon atom occupies the center of an idealized tetrahedro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and metabolism. Over the last decades of the 20th century, biochemistry has become successful at explaining living processes through these three disciplines. Almost all areas of the life sciences are being uncovered and developed through biochemical methodology and research.Voet (2005), p. 3. Biochemistry focuses on understanding the chemical basis which allows biological molecules to give rise to the processes that occur within living cells and between cells, Karp (2009), p. 2. in turn relating greatly to the understanding of tissues and organs, as well as organism structure and function. Miller (2012). p. 62. Biochemistry is closely related to molecular biology, which is the study of the molecular mechanisms of biological phenom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |